topic 2 C) D) - biological molecules and movement of substances
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
24 Terms
chemical elements in carbohydrates
carbon, oxygen, hydrogen
chemical elements of lipids
carbon, oxygen, hydrogen
chemical elements of proteins
carbon, oxygen, hydrogen and nitrogen (sometimes sulphur)
what are carbohydrates
small simple sugars or more complex larger molecules
monosaccharide = simple sugar e.g. glucose, fructose
disaccharide = two monosaccharides joined together e.g. maltose from 2 glucose
large polysaccharide = lots of monosaccharides joined together e.g. starch, glycogen, cellulose from glucose (insoluble storage molecules)
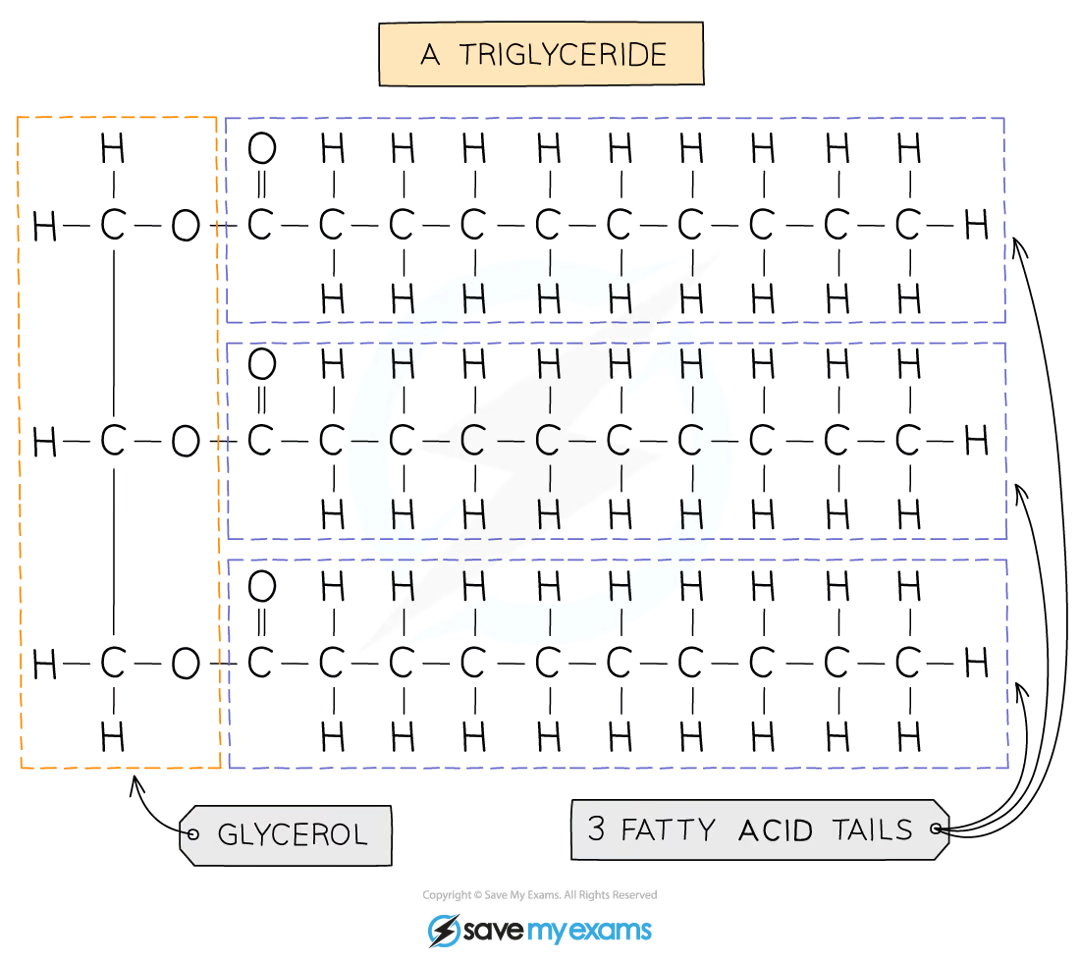
what are lipids
made up of triglycerides
basic unit is one glycerol molecule chemically bonded to three fatty acid chains
divided into fats (solids at r.t) and oils (liquids)
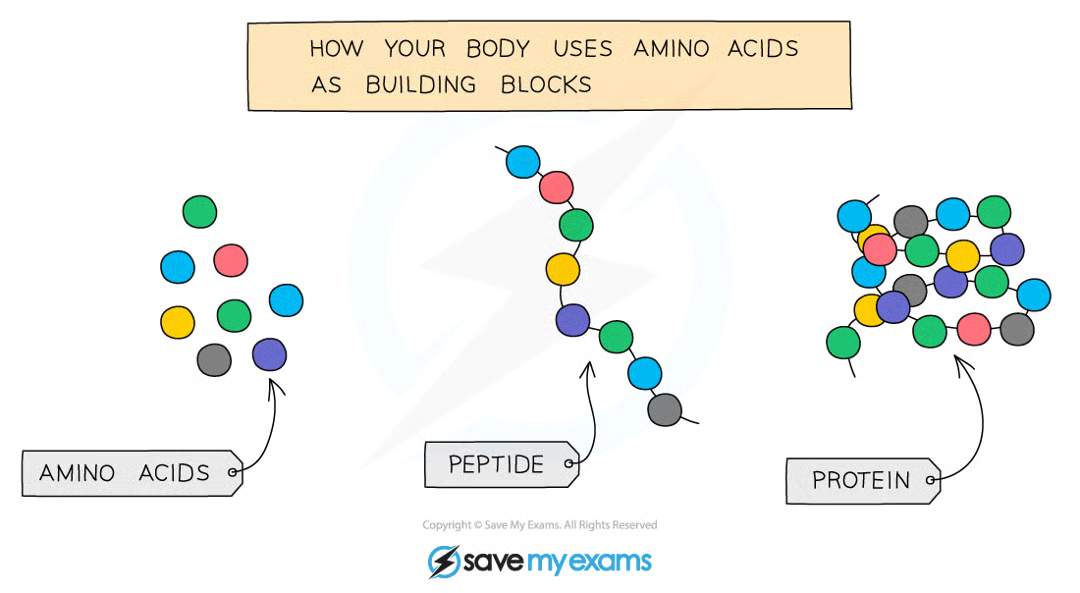
what are proteins
formed from long chains of amino acids
different proteins have different a.a sequences and therefore shapes
shape of a protein determines its function
e.g. enzymes, haemoglobin, ligaments, keratin
how to prepare a sample of food for food tests
break up food using pestle and mortar
transfer to test tube and add distilled water
mix with water by stirring with glass rod
filter mixture using funnel and filter paper, collect the solution
test for glucose
add benedicts solution
heat in boiling water bath for 5 minutes
take out and observe colour
positive = from blue to orange/brick red
test for starch
add drops of iodine solution to food sample
positive = from orange/brown to blue/black
test for protein
add drops of Biuret solution
positive: colour change from blue to violet/purple
test for lipids
mix food sample with 4cm³ of ethanol and shake
allow time for sample to dissolve in ethanol
strain into another test tube
add ethanol solution to an equal volume of cold distilled water
positive test forms cloudy emulsion
what are enzymes
proteins that act as biological catalysts to speed up rate of reaction without being changed or used up
biological because they are made in living cells
essential to maintain reaction speeds of all metabolic reactions at a rate that can sustain life
how do enzymes work
enzymes are specific to one particular substrate because the active site of the enzyme (where the substrate attaches) is complementary to the shape of the substrate
enzymes and substrates randomly move about in solution
when an enzyme and its complementary substrate collide, an enzyme-substrate complex forms and reaction occurs
a product forms from the substrate and is released from the active site
factors affecting enzyme action
temperature
pH
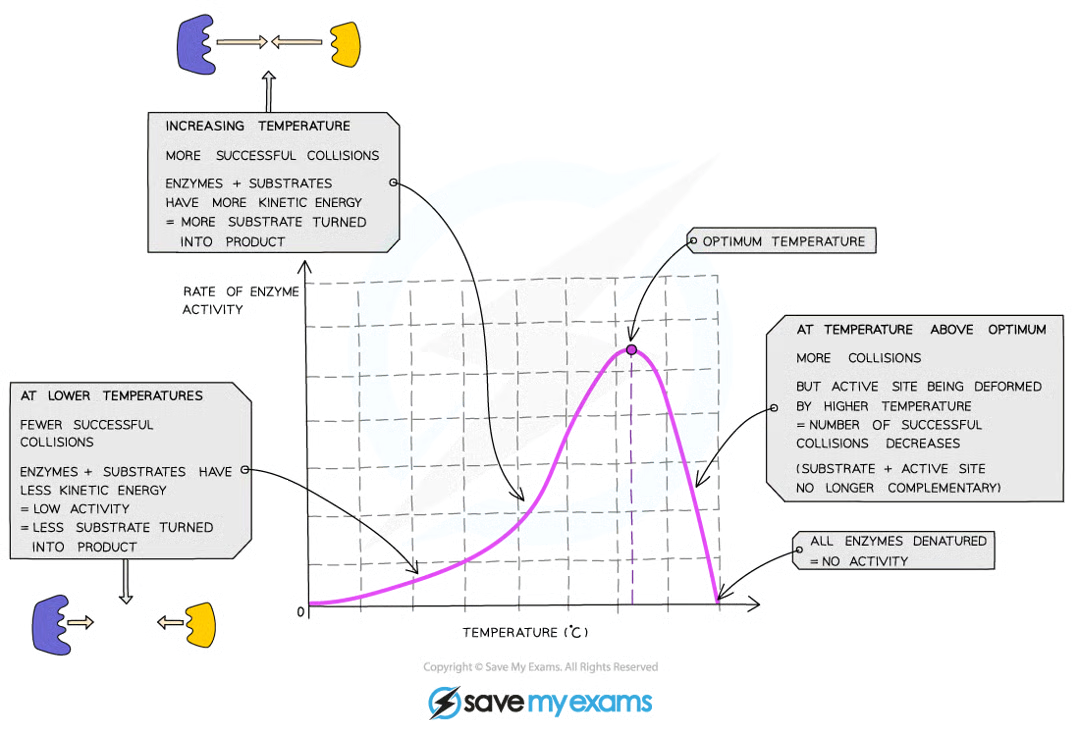
how does temperature affect enzyme action
enzymes work fastest at their optimum temperature (37 in the human body)
heating to higher temperatures will break the bonds that hold the enzyme together and it will lose its shape and denature
substrates cannot fit into denatured enzymes as the shape of the active site is lost
increasing temp towards optimum increases activity of enzymes as more kinetic energy means molecules move faster and therefore collide more with substrate molecules
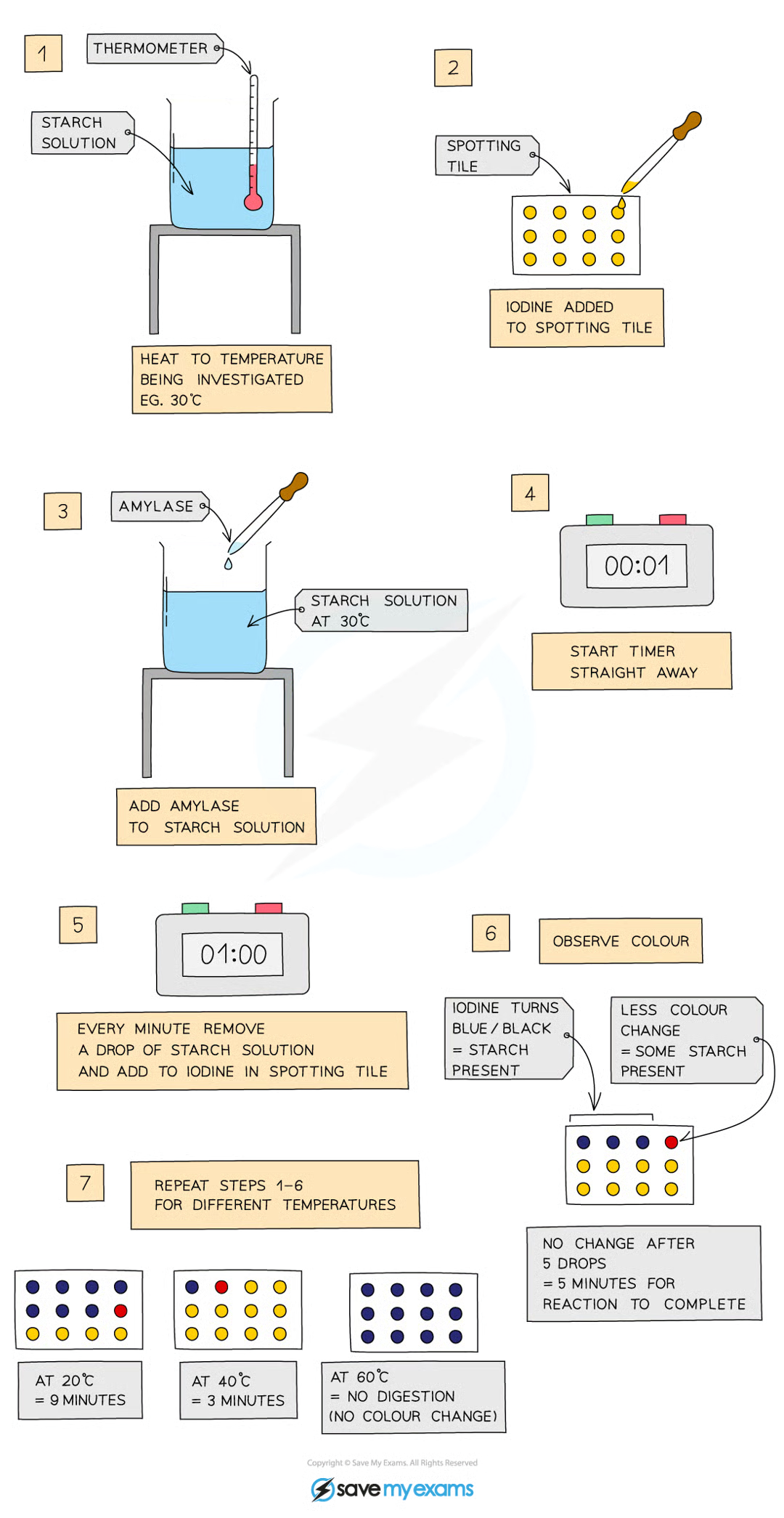
practical for effect of temperature on enzymes
amylase is an enzyme that digests starch into maltose
add 5cm³ starch solution to a test tube and heat to a set temperature
add drop of iodine to each of the wells of a spotting tile
use a syringe to add 2cm³ amylase to the starch solution and mix
every minute, transfer a droplet of solution to a new well of iodine which should turn blue-black
repeat until the iodine stop turning blue-black (means amylase has broken down all starch)
record time taken for the reaction to be completed
repeat for a range of temps from 20-60
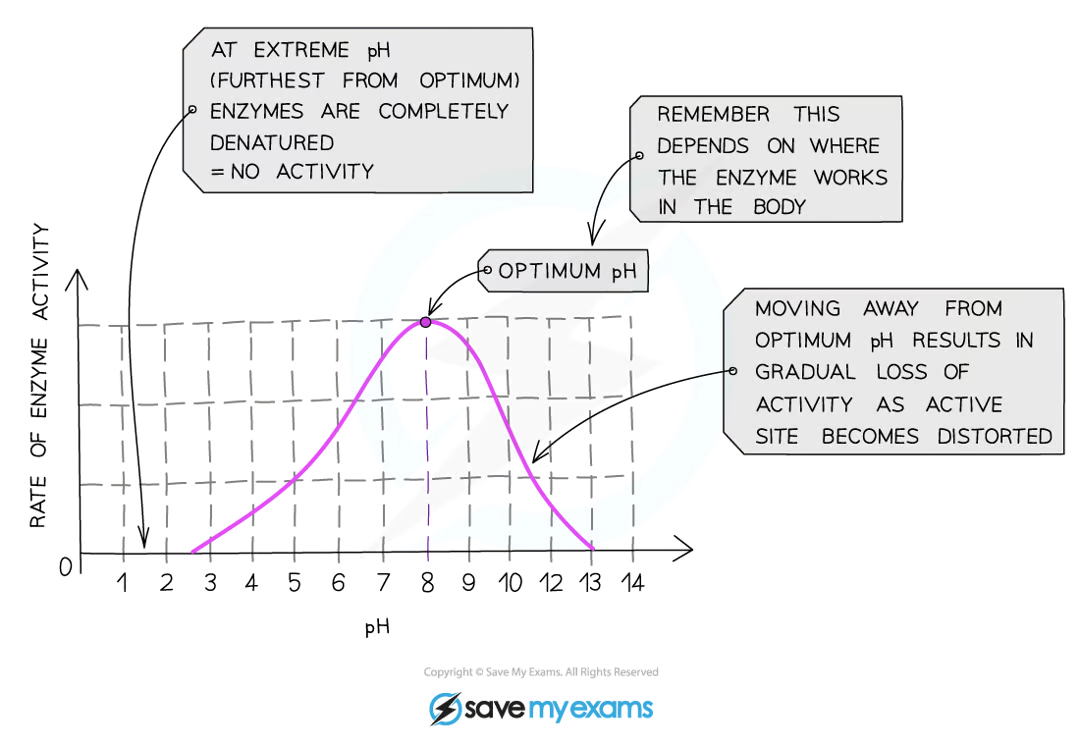
how does pH affect enzyme action
if pH is too high or low, the bonds that hold amino acid chain together to make the protein can be destroyed, changing shape of active site
moving too far away from optimum pH will cause denaturing
optimum for most is 7, however some eg. stomach will have lower and some in alkaline e.g. duodenum will have higher
practical investigating effect of pH on enzymes
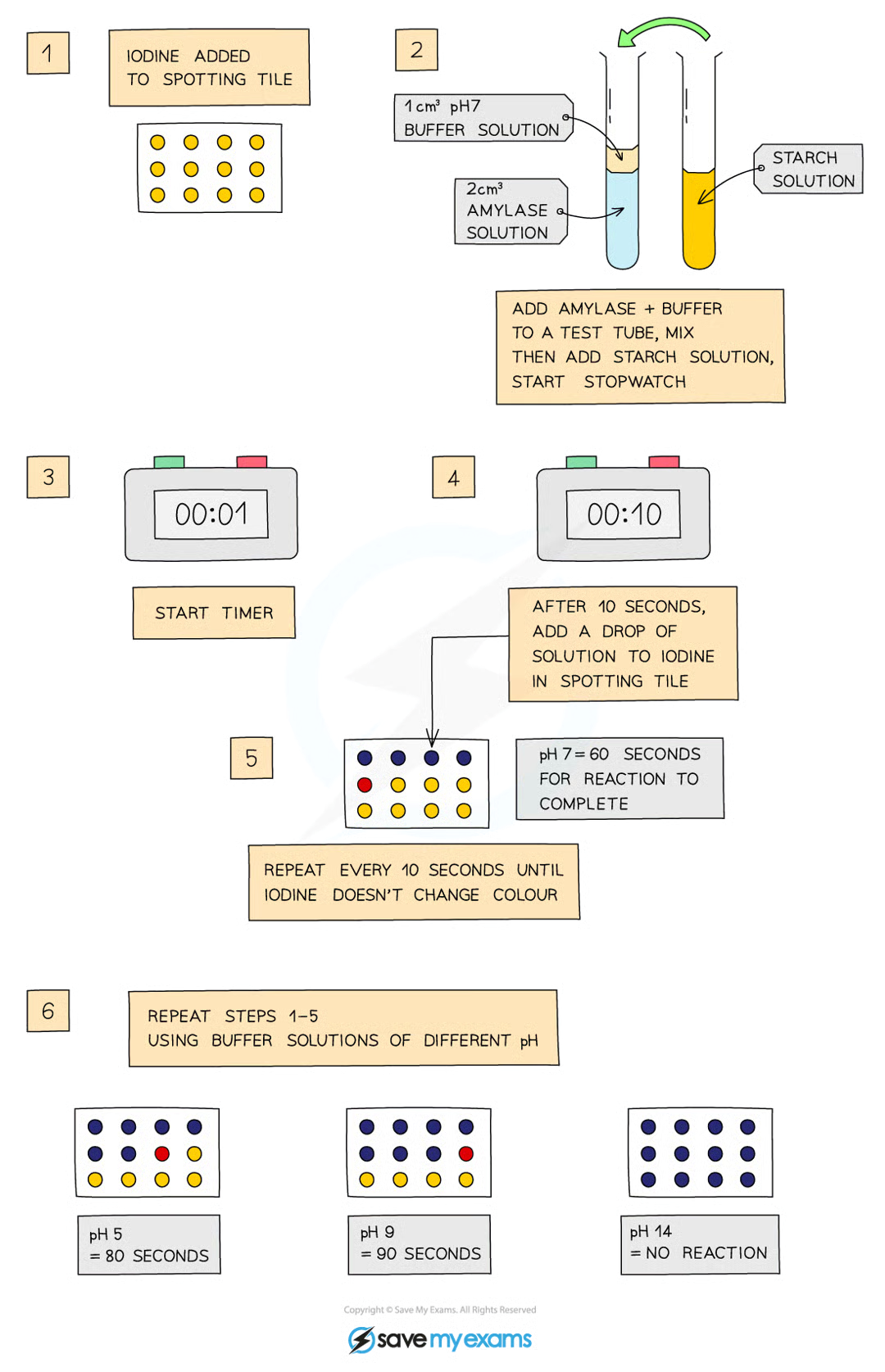
amylase digests starch into maltose
add a drop of iodine to each of the wells of the spotting tile
use a syringe to place 2cm³ of amylase into a test tube
add 1cm³ buffer solution at pH2 to the test tube using a syringe
use another test tube to add 2cm³ of starch solution to amylase and buffer solution, then start stopwatch whilst mixing using pipette
every 10 seconds transfer a droplet of solution to a new well of iodine solution (should turn blue-black)
repeat every 10 seconds until iodine stops changing colour
record time taken for reaction to be completed
repeat with different pH buffers from pH3-7
what is diffusion
the movement of molecules from a region of higher concentration to a region of lower concentration
moves down concentration gradient, molecules move with random movement
results in even concentration throughout
molecules move into or out of living cells by diffusion when they cross the cell membrane (partially permeable)
allows living organisms to: gain nutrients in digestive system, gain oxygen in lungs, remove waste products in lungs and kidneys
what is osmosis
movement of water molecules from a region of higher water concentration (dilute solution) to region of lower water concentration through a partially permeable membrane
diffusion of water moving down concentration gradient
without a cell wall, osmosis can have severe effects on animal cells
in lower water concentration, cell loses water and shrivels
in higher water concentration, cell gains water and eventually bursts
due to cell wall, plant cells are protected from bursting
in lower water concentration, cell loses water, vacuole shrinks, cell membrane pulls away from wall and makes cell flaccid
in higher water concentration cell gains water, vacuole expands, membrane pushes against cell wall, making cell turgid
what is active transport
The movement of particles across a cell membrane from a region of lower concentration to a region of higher concentration
energy is needed as particles move against concentration gradient
a.t across cell membrane involves protein carrier molecules that are in the cell membrane
e.g. absorption of products of digestion into the bloodstream from the lumen to the small intestine
absorption of mineral ions form soil to root hair cells
factors influencing diffusion
SA:V ratio - larger SA:V ratio = faster rate of substance movement
diffusion distance - smaller the distance, faster the transport
temperature - higher temp = faster movement
concentration gradient - greater difference in concentration, faster movement
practical investigating factors affecting diffusion
beetroot: cells have dark purple/red pigment
heating above 45 can damage cell membrane, causing pigment to leak out
speed at which pigment leaks tells rate of diffusion
use a knife to cut 2 equally sized cubes of beetroot
rinse
put 5cm³ water into 2 test tubes A and B
keep A at room temp and transfer test tube B to hot water bath at 90
leave test tube for 2 mins, then add a piece of beetroot
after 10 mins, observe colour of liquid in both
higher temp = more leaked pigment
practical investigating factors influencing osmosis
prepare a range of sucrose solutions ranging from 0mol/dm³ to 1
set up 6 labelled test tubes w 10cm³ of each of the sucrose solutions
use knife cork borer and ruler to cut 6 equal sized cylinders of potato
blot each one with paper towel and weight
put 1 piece into each concentration
after 4 hours, remove, blot and reweigh
potato in distilled water will gain most mass due to high conc. gradient, causing water to move in and make cell firm
potato in strongest sucrose solution will lose most mass as water moves out of cells by osmosis, making cell flaccid