Earth Systems Lecture 5
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
20 Terms
Rocks, Minerals and Melting
Classification and Formation of Rocks
Igneous
–Rocks that have formed by the solidification of hot, mobile
material (magma).
Metamorphic
–Rocks that have formed from other rocks by exposure to unstable
conditions of pressure and temperature without inducing melting.
Sedimentary
–Rocks that have formed by the reconstitution of other rocks or
rock fragments (sediment), or by low temperature precipitation or
by biogenic processes
Rocks are made of minerals/mineral fragments
A mineral is naturally occurring solid, usually inorganic, a defined (but slightly variable) chemistry, an ordered internal structure.
Mineral Classes
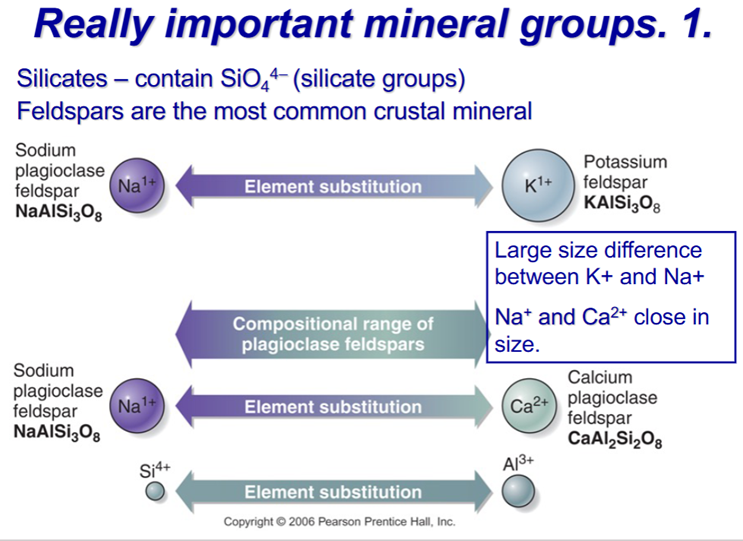
Silicates - most minerals in the crust
Oxides - O2- (e.g. FeO, Fe2O3, Fe3O4)
Sulphides - S2- (e.g. PbS, FeS2)
Sulphates - SO42- (e.g. CaSO4.2H2O)
Halides or salts - Cl- and F- (e.g. NaCl, CaF2 )
Carbonates - CO32- (e.g. CaCO3, CaMg(CO3)2)
Native metals - Cu, Au, Sn
Eight of the top ten elements are cations (have +ve charge)
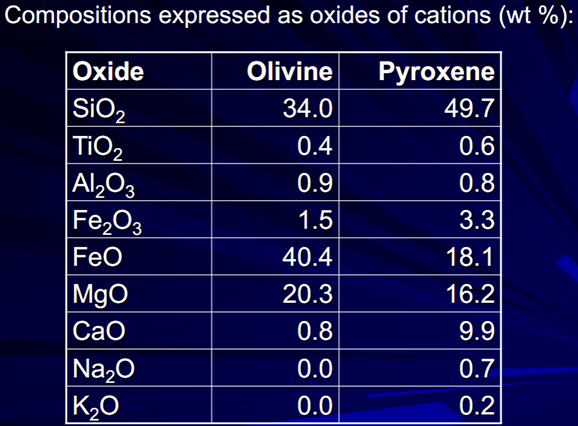
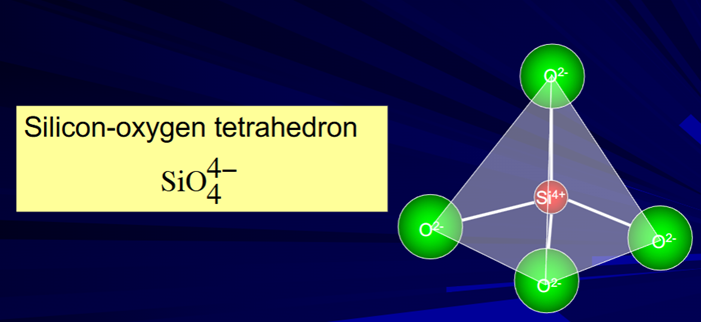
Oxygen is the dominant anion therefore rock-forming mineral are dominated by OXIDES but the minerals are actually built up from tetrahedra made from Si and O – and the metal cations give charge balance
Characteristics
Lustre- How it reflects light e.g. glassy, dull, metallic, etc.
Crystal faces- characteristic shapes
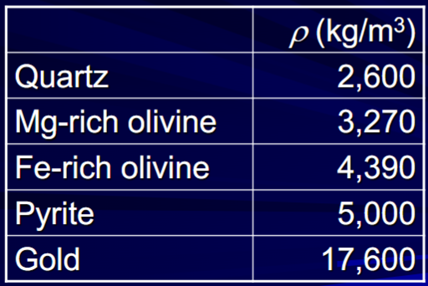
Density (r) – (g/cm3 or kg/m3)
Density can be predicted by adding the masses of the component oxides. Density has many physical implications for mineral movement
Cleavage – the way a mineral “breaks” into smaller bits of characteristic shape.
–flat cleavage faces
–smaller pieces resemble larger ones
Fracture- some minerals break irregularly. Fragments have no characteristic shapes
Colour and shape
–a range of colours and/or shapes
–Generally shape and colour are not diagnostic
Streak– the colour of a mineral scratched on unglazed porcelain. Two examples of the same mineral may vary in colour, but streak will be the same
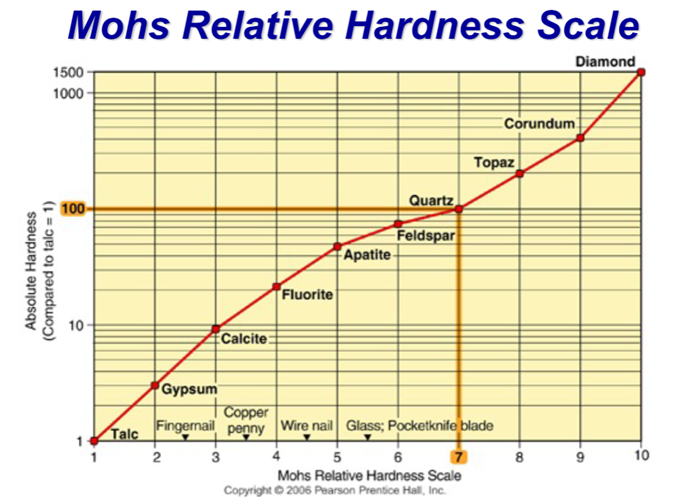
Characterising Hardness
–Resistance to scratching
–Hard minerals scratch softer ones
–Calcite is softer than quartz
Determining Colour
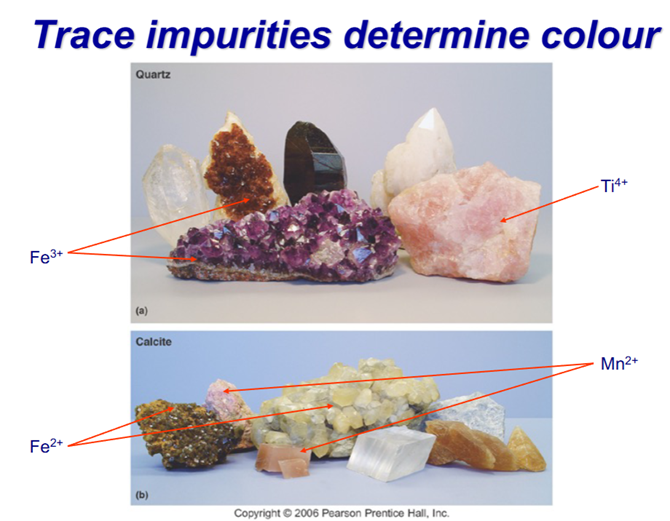
Streak– the colour of a mineral scratched on unglazed porcelain. Two examples of the same mineral may vary in colour, but streak will be the same
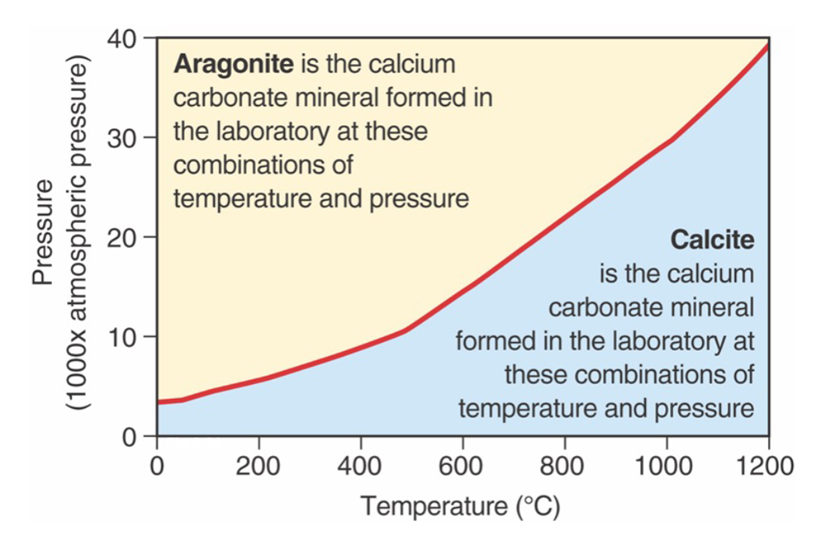
Polymorphs
Calcium, carbon, and oxygen form calcium carbonate
Two mineral forms: Calcite – the “common” arrangement of CaCO3. Aragonite – secreted by shelled organisms, and abiologically under certain conditions
The atomic structure of minerals
Chemical bonds
–All form due to interaction of electrons
–The outer layer of electrons that interact are called valence electrons
Types of bonds
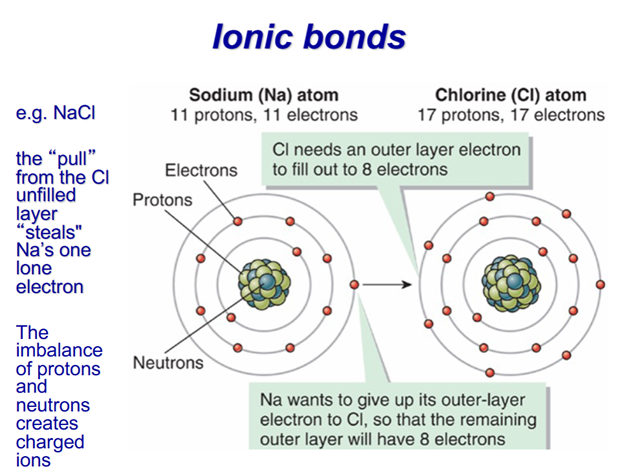
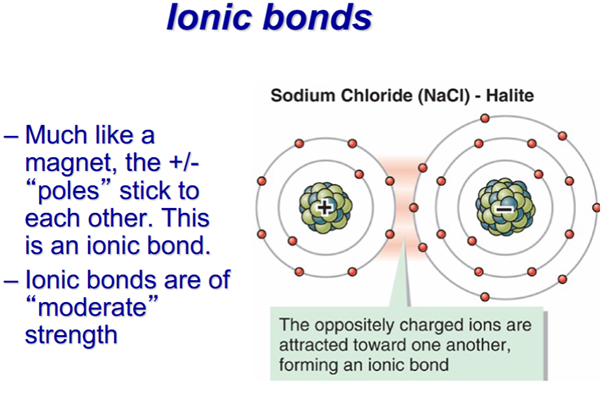
–Ionic
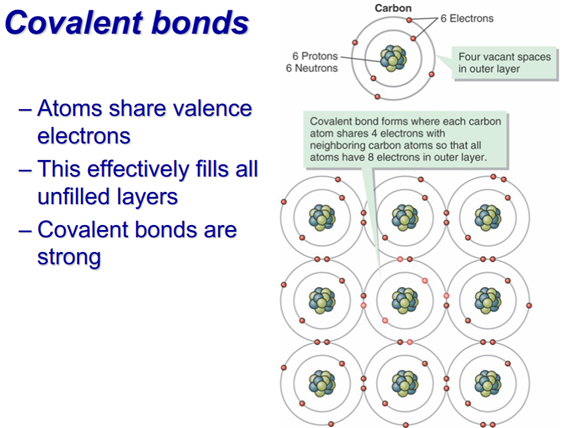
–Covalent
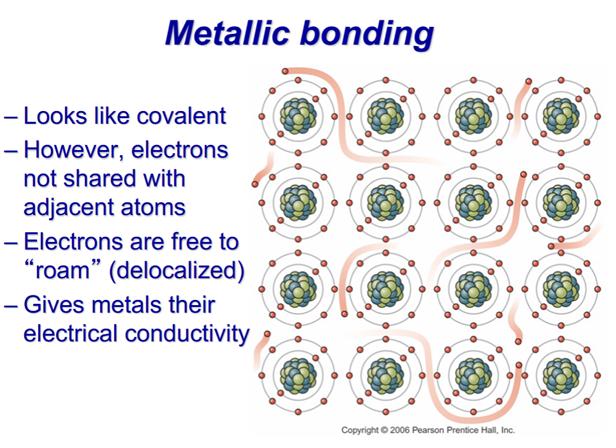
–Metallic
Van der Waals Forces
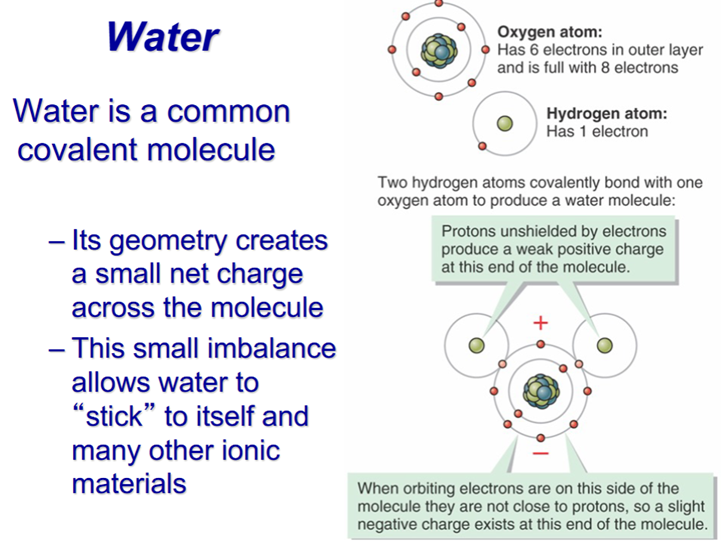
Unequal electron distribution around atoms or molecules creates a weak charge asymmetry:
One side is more positive, the other more negative
Can create a weak attractive force
This is very much like the polarity of water molecules
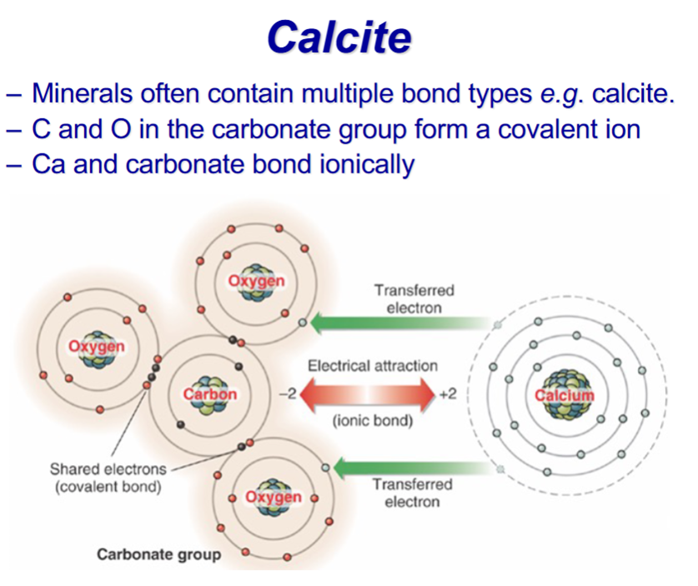
Calcite is ionically soluble in water (giving Ca2+/CO32– in solution) But acid will break the covalent carbonate down and release CO2
Atomic Structure Determines Physical Characteristics
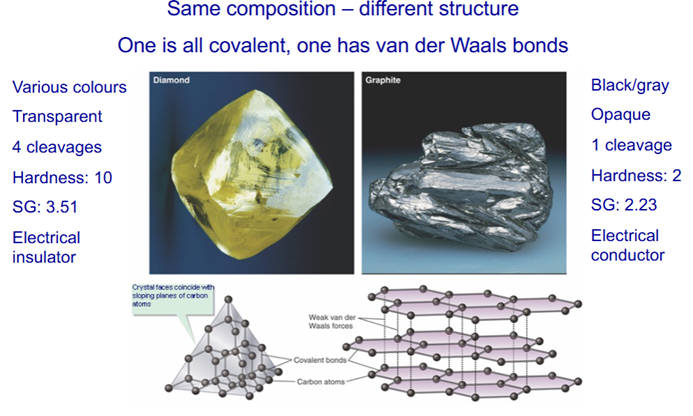
Bond type and order affects mineral hardness
–Quartz – all covalent, Hardness (H): 7
–Calcite– covalent and ionic, H: 3
Ca2+ – hardness determined by the weaker ionic bond
-Halite (NaCl), in ionic form Na+, H: 2.5. For ionic compounds, hardness is proportional to charge
-Graphite – covalent and van der Waals, H: 2
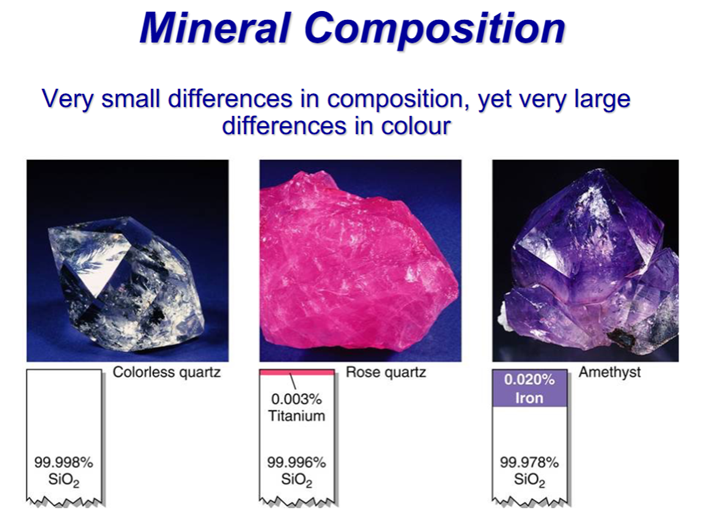
Small variations in composition can cause different colouring in a mineral.
The type of bond and distance between bonded atoms determines mineral hardness.
-Bond strength: covalent > ionic > van der Waals
-For covalent: closer = stronger
-For ionic: strength is proportional to charge
Density of minerals is greater for larger or more closely packed atoms.
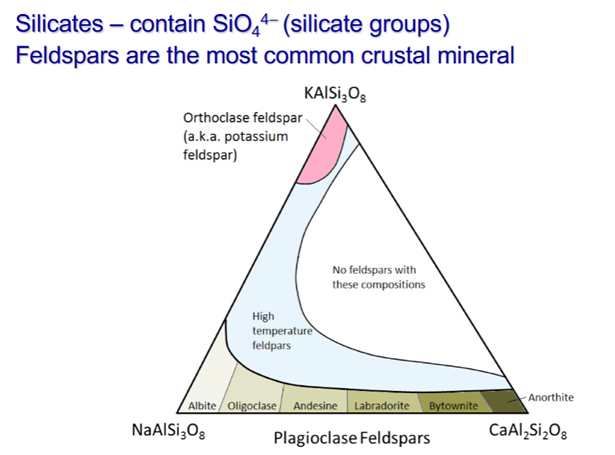
Important Mineral Groups


Igneous Rock
-Crystallised from a melt
-Comprised of multiple grains of one or more mineral phases and/or glass
-Textural information, e.g. crystal form, crystal size, holes from gas bubbles
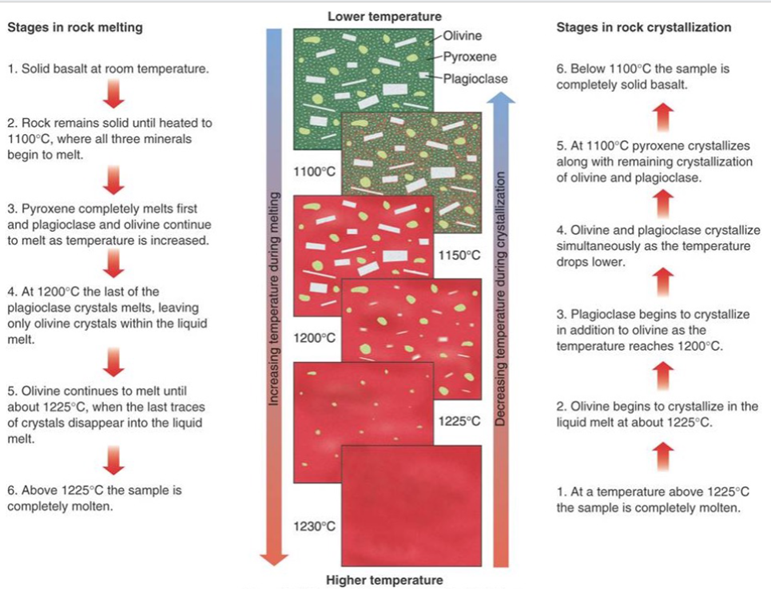
Why do magmas solidify?
If temperature is lowered enough, a magma will solidify into a rock
Silicate minerals crystallise / melt across a temperature range from 800oC to around 1900oC (at sea level). This is known as the crystallisation interval or melting interval.
-Increasing pressure increases melting temperature. At sea level a Mg-olivine melts at 1890oC, at 100 km depth it melts at 2050oC
-Mixtures melt at a lower temperature than lone mineral. Olivine mixed with pyroxene and plagioclase melts at ~1200oC (at sea level)
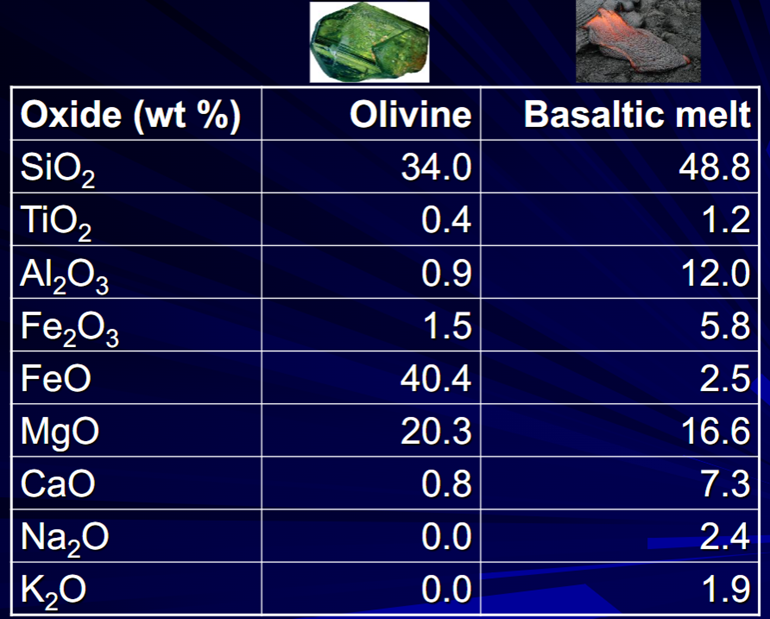
Different mineral phases crystallise from a melt at different temperatures, (or conversely, they melt from a solid rock at different temperatures).
The minerals that crystallise have a different composition from the residual melt. Therefore, if the crystals are separated from the melt— fractional crystallisation—we are left with a magma with a different composition from the parent magma.
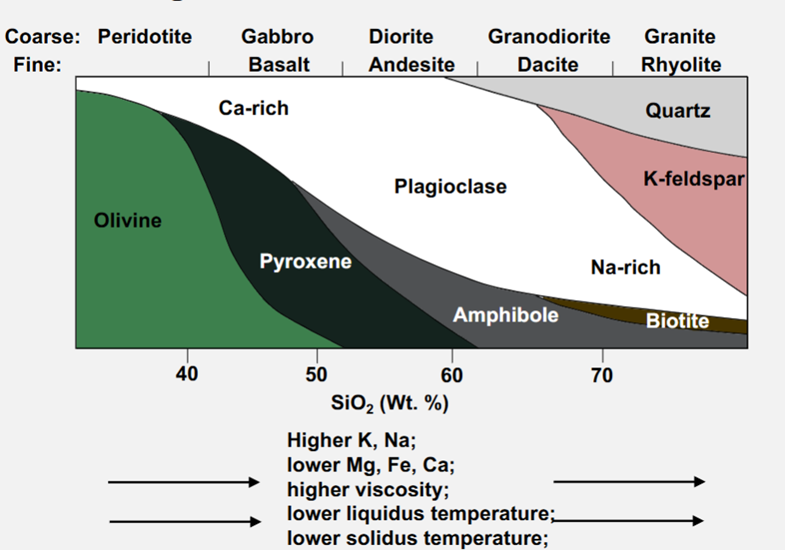
Diversity of Igneous Rock Compositions
Fractional crystallisation: As minerals crystallise out, the remnant melt composition changes
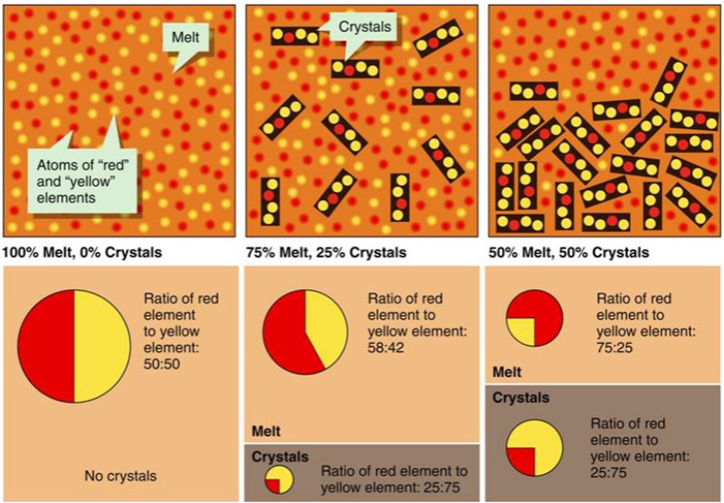
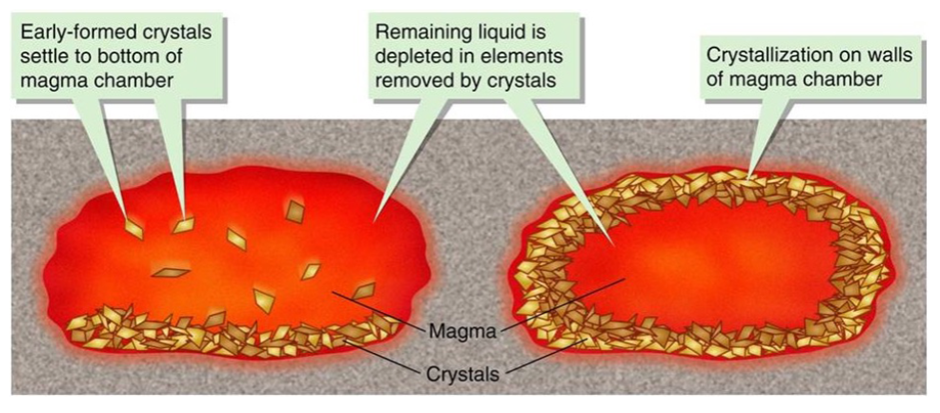
So these first-to-form crystals may settle (if denser), or stay where they form—on the walls (cooling occurs from outside in) to form vertical or concentric layers.
Extent of Melting
-When a rock melts completely (temperature high enough to melt all minerals) then the magma has the same composition as the parent rock.
-Incomplete melting or crystallisation can yield different end rocks and liquids from the same starting composition
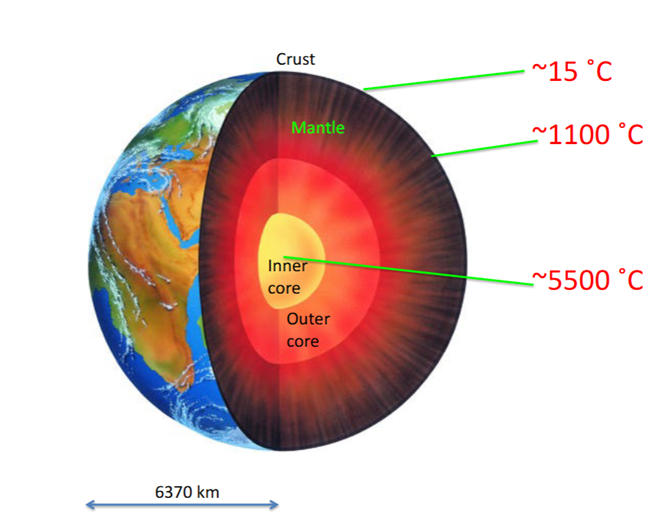
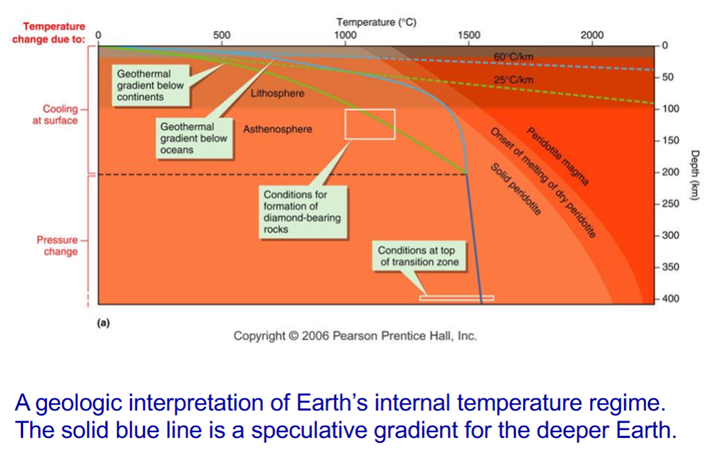
Geothermal Gradient
Earth’s temperature profile- temperature increases as you go deeper
Temperatures increases rapidly at first (uppermost 10–50 kilometers)
-Beneath continents: 25–30 oC km-1
-Under the oceans: ~60 oC km-1
If this continued within Earth (say at 30oC/km) the core would have to be at 200,000oC. This is unreasonable.
-Therefore, the gradient must decrease with depth. The mantle low-velocity zone must be partially molten at depths of 75–100 km, suggesting a temperature of 1300oC. Diamonds are created at ~1000–1200oC at ~100–150 km depth; therefore the gradient under continents is less than 10oC/km. Temperatures of 1450 ± 150oC are needed to transform olivine into denser minerals at 410 km; this means a gradient of only 3.5oC beneath oceans.
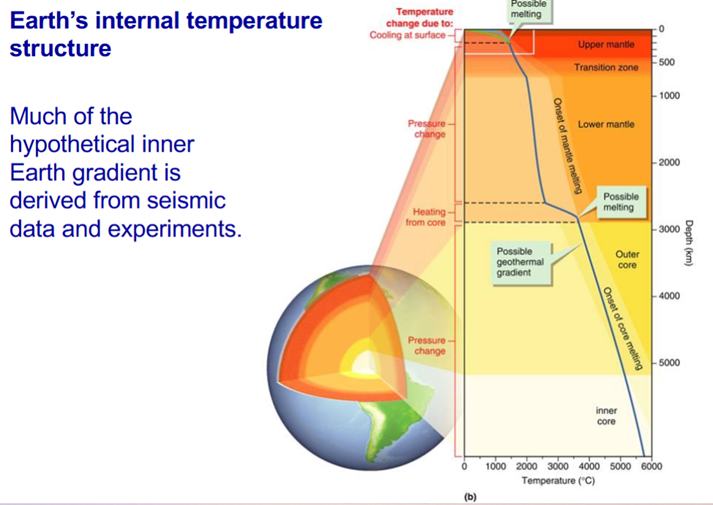
Why is the interior hot?
Heat must be generated within Earth in order to account for its high internal temperature. This is due in part to radioactive decay of potassium, uranium, and thorium in silicate minerals. However, their volume is not sufficient in the mantle and core to account for Earth’s internal heating. This missing heat is from crystallisation of iron.
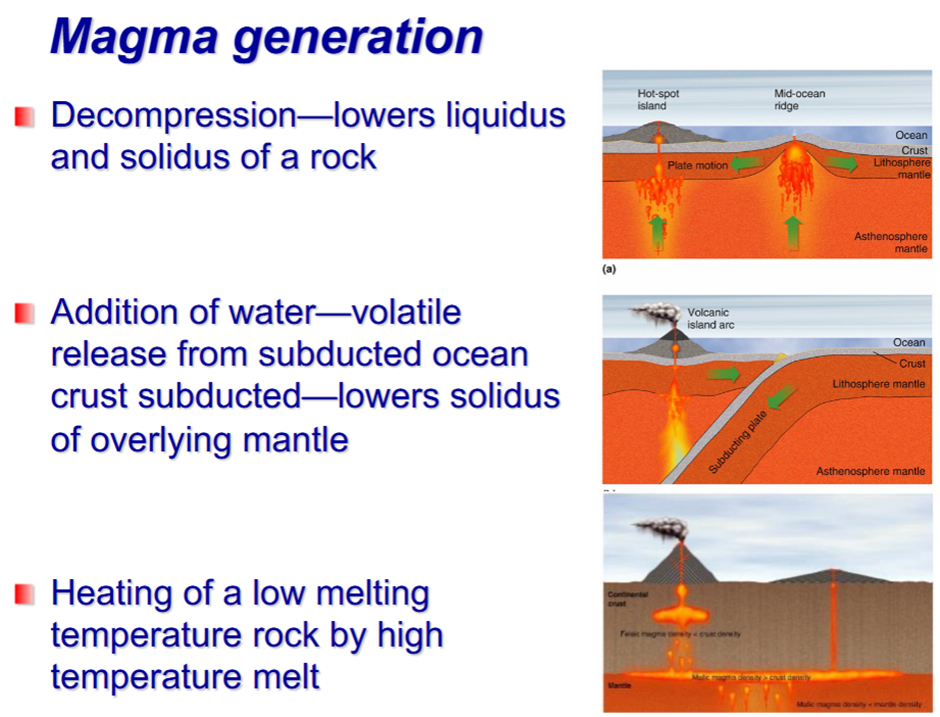
Generation of Magma
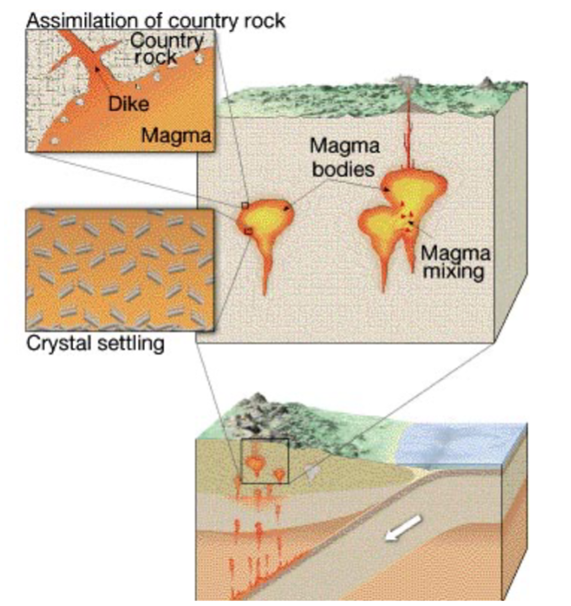
Other things that complicate the picture
Magma assimilation: as magma rises through surrounding rock it melts; parts are
incorporated into the melt.
Magma mixing: when a new “pulse” of magma injects into an intrusive body it may mix,
forming a magma of different composition than either.
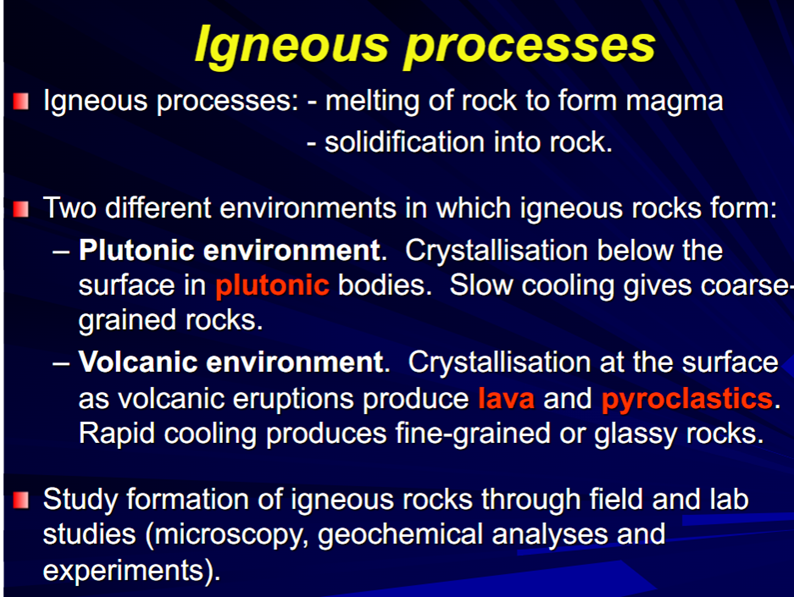
Igneous Processes
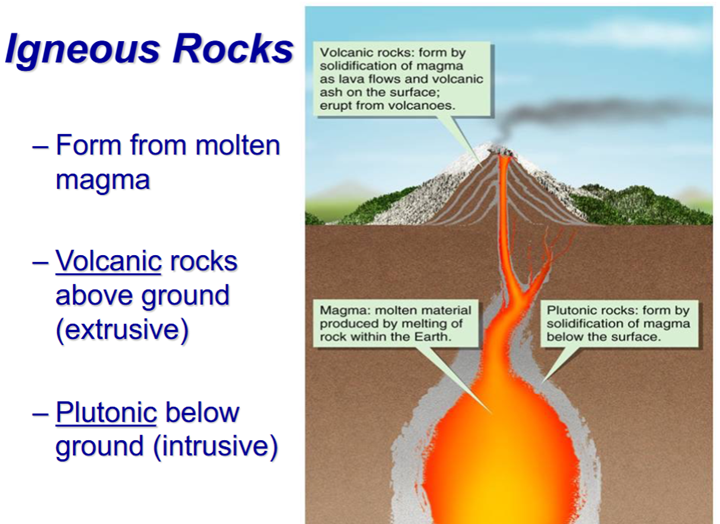
Volcanic Rock
From flowing lava at the surface, or from exploding/ejected materials that tend to form glass
Plutonic rocks
Forms from magma that cools below Earth’s surface
Has crystals that are big enough to see with the unaided eye