CHEMISTRY FLASCHARDS INVOLVING REMEMBERING DIAGRAMS
0.0(0)
Card Sorting
1/5
There's no tags or description
Looks like no tags are added yet.
Last updated 8:53 PM on 2/10/26
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
6 Terms
1
New cards
this
2
New cards
3
New cards

4
New cards
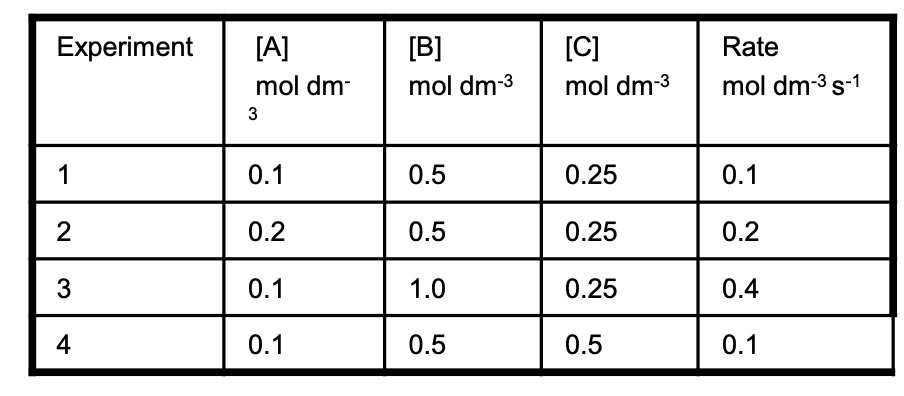
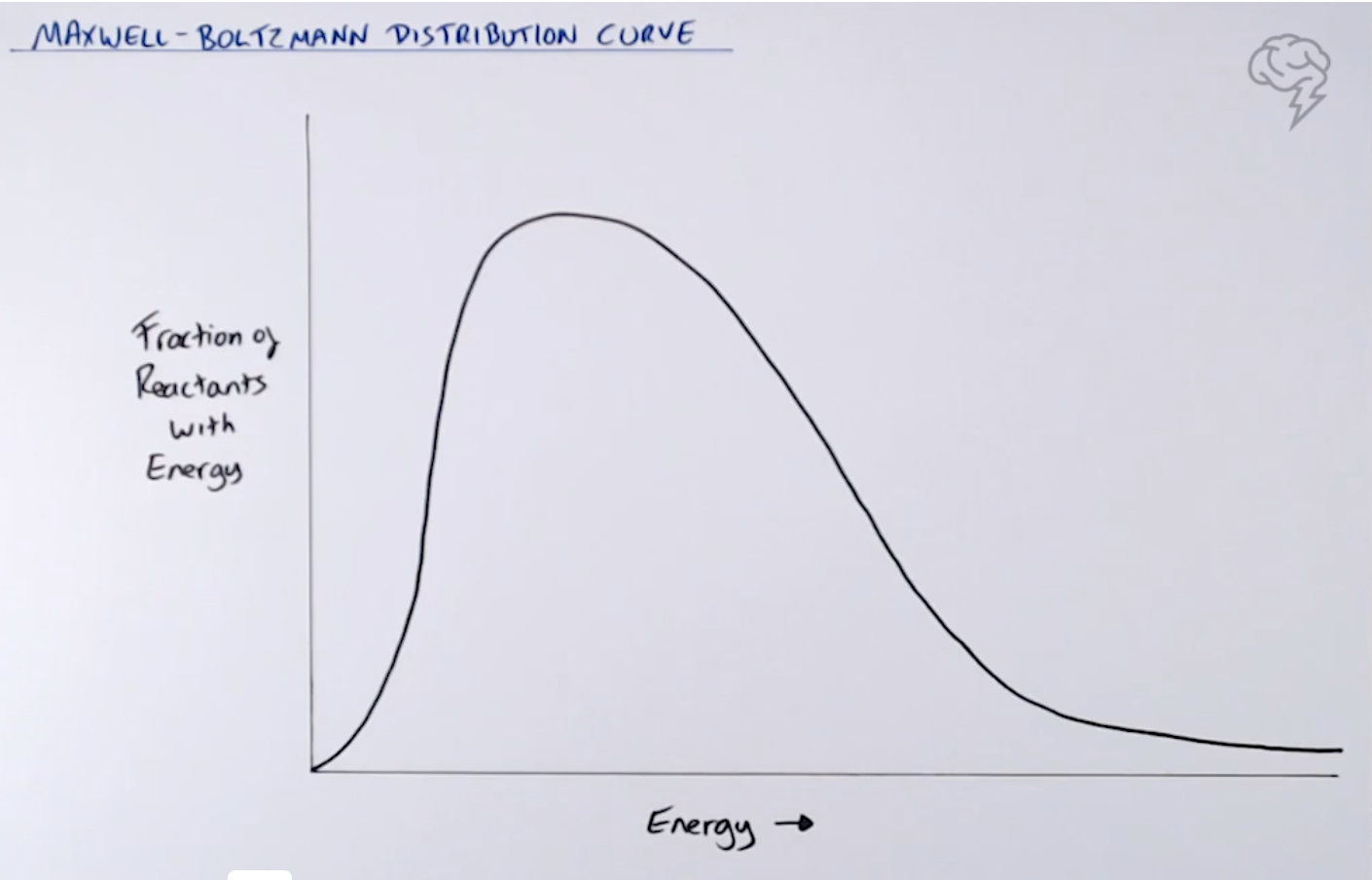
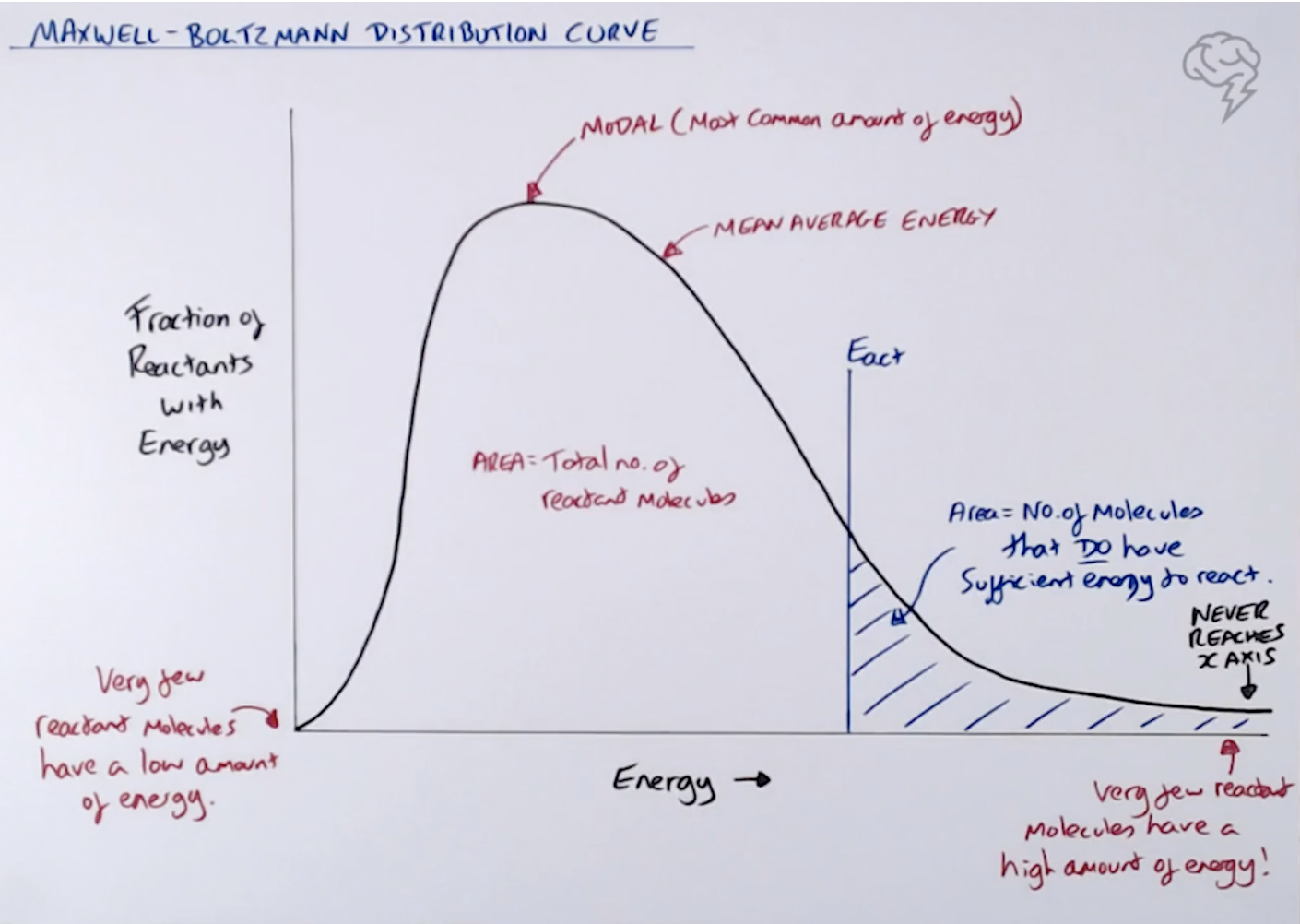
Deduce the rate equation for the following reaction, A+ B+ 2C ——> D + 2E, using the initial rate
data in the table

5
New cards
6
New cards
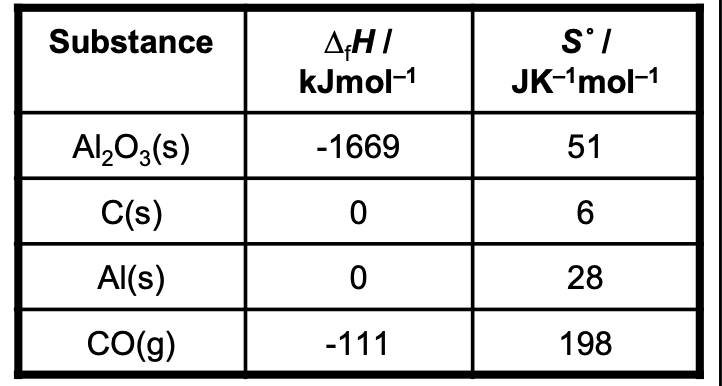
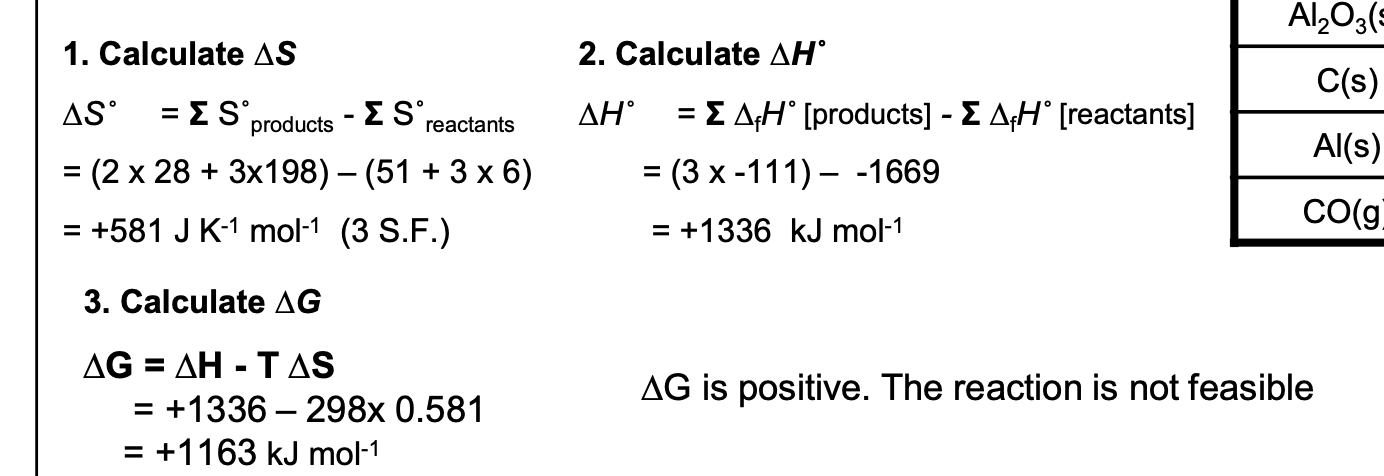
Data for the following reaction, which represents the
reduction of aluminium oxide by carbon, are shown in the table.
Al2O3(s) + 3C(s) → 2Al(s) + 3CO(g)
Calculate the values of ∆H, ∆S and ∆G for the above reaction at 298 K