Edexcel Physics IGCSE - Radioactivity and Particles
1/35
Earn XP
Description and Tags
Flashcards covering key terms and concepts from the Edexcel Physics IGCSE lecture on Radioactivity and Particles.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
36 Terms
Atom
A basic unit of matter consisting of a nucleus made of protons and neutrons, surrounded by electrons.
Isotope
Forms of an element’s atom with the same number of protons but a different number of neutrons.
Radioactive decay
The spontaneous transformation of an unstable nucleus into a more stable one by the release of radiation.
Alpha particle
A heavy nucleus emits an alpha particle, which is a helium nucleus consisting of 2 protons and 2 neutrons.
Beta particle
A neutron turns into a proton which then emits an electron
Gamma radiation
Electromagnetic radiation emitted from a nucleus, which is lowly ionising and highly penetrating.
Half-life
The time taken for half the nuclei in a sample to decay, or for the activity of a radioactive source to halve.
Background radiation
Radiation from external sources that is always present, such as cosmic rays and radioactive rocks.
Geiger-Muller tube
A device that detects radiation by producing an electrical pulse each time radiation is absorbed.
Nuclear fission
The process of splitting a nucleus into smaller nuclei, releasing energy and neutrons.
Nuclear fusion
The process of fusing two smaller nuclei to form a larger nucleus, releasing energy.
Contamination
When a radioactive source is introduced into or onto an object.
Irradiation
When an object is exposed to a radioactive source from outside without becoming radioactive itself.
Control rods
Materials used in a nuclear reactor to absorb neutrons and regulate the fission process.
Coolant
A substance used in a nuclear reactor to remove heat and prevent overheating.
Charachteristics of an Alpha particle
They are highly ionizing and weakly penetrating particles that consist of two protons and two neutrons, equivalent to a helium nucleus.
What can stop an Alpha particle?
A thin sheet of paper
Why are Alpha particles highly ionizing and weakly penetrating?
They are highly ionizing because they have a positive (+2) charge and so when they pass next to an atom they can attract electrons in the outer shell and rip them off. They are weakly penetrating because they have a very large size compared to other kinds of radiation so they can hardly pass through any materials
Charachteristics of a Beta particle
They are moderately ionizing and moderately penetrating
What can stop a Beta particle?
A think sheet of aluminium
Why are Beta particles moderately ionizing and moderately penetrating?
They are moderately ionzing because it consists of high speed electrons and when these pass next to an atom, can collide with the electrons in the outer shell and rip them of. They are moderately penetrating because electrons have a relatively low mass and speed to pass trhough materials
Charachteristics of a Gamma particle
Gamma particles are electromagnetic radiation with no mass or charge, highly penetrating, and emitted from the nucleus during radioactive decay. They have high energy and can travel long distances through matter. It has a relatively low ionizing ability
What can stop a Gamma particle?
A thick sheet (several centimeters) of lead or several centimeters of concrete.
Why are Gamma particles lowly ionizing and highly penetrating?
Gamma particles are considered lowly ionizing due to their relatively small mass and charge, leading to less interaction with matter than alpha particles. Because of this, they cannot easily rip off electrons from the outer shell of an atom. They are highly penetrating because with no mass and a very high speed, they can travel through materials more easily than heavier charged particles.
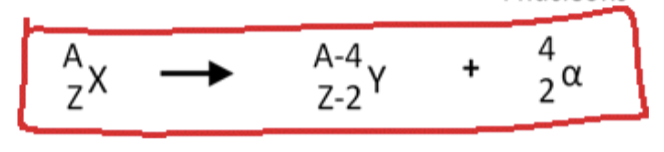
Alpha decay
is a type of radioactive decay wherein an unstable nucleus emits an alpha particle, consisting of two protons and two neutrons. This process reduces the atomic mass of the original nucleus and results in a different element.
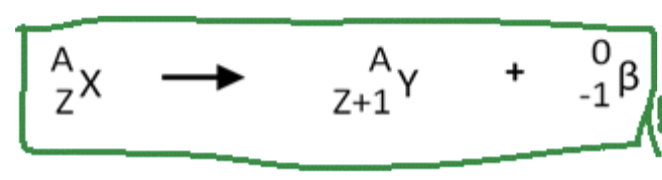
Beta Decay
is a type of radioactive decay in which an unstable nucleus emits a beta particle, which is an electron or positron, leading to a transformation of a neutron into a proton or vice versa. This process alters the element's atomic number while keeping the atomic mass nearly the same.
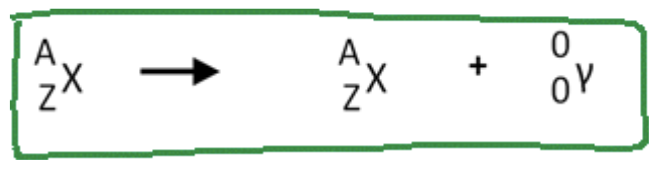
Gamma decay
is a type of radioactive decay where a nucleus emits gamma rays, which are high-energy photons. This process occurs after alpha or beta decay to release excess energy and stabilize the nucleus without changing its mass or atomic number.
Neutron radiation
is a type of radiation consisting of free neutrons that are ejected from a nuclear reaction. Neutron radiation can cause significant damage to materials and biological tissues, and it is often encountered in nuclear reactors and during certain types of radioactive decay. The nucleus becomes a new isotope of the original element according to the following equation: ZAX —> (X-A) + 1n
Photographic film
is a light-sensitive material used to capture images, commonly employed in cameras and other photographic equipment. It reacts to radiation, including gamma and beta particles, producing a visible image upon development. The more radiation absorbed by the film, the darker it gets (initially white)
Sources of background radiation
From space (cosmic ray include high energy particles penetrating the atmosphere)
From Earth (radiactive rocks which give off radioactive radon gas, food and drink which contains radioactive isotopes (e.g. carbon 14), fallout from nuclear weapons testing, nuclear power plants which produce radioactive waste, medical sources such as x-rays from MRI scanners)
Becquerels mesaures what?
the activity of a radioactive source is the number of decays which occur per unit time
Uses of radioactivity in industry
Smoke detectors: Long half-life alpha emitters are used in smoke detectors. Alpha particles cause a current in the alarm. If smoke enters the detector, some of the alpha particles are absorbed and the current drops, triggering the alarm.
Thickness monitoring: Long half-life beta emitters can be used for thickness monitoring of metal sheets. A source and receiver are placed on either side of the sheet during its production. If there is a drop or rise in the number of beta particles detected, then the thickness of the sheet has changed and needs to be adjusted.
Uses of radioactivity in medicine$
Sterilisation of equipment: Gamma emitters are used to kill bacteria or parasites on equipment so it is safe for operations (this means they can be sterilised through their protective packaging to eliminate the risk of contamination).
Diagnosis and treatment: Short half-life gamma emitters such as technetium-99m are used as tracers in medicine as they concentrate in certain parts of the body. The half-life must be long enough for diagnostic procedures to be performed, but short enough to not remain radioactive for too long. Other gamma emitters such as cobalt-60 can be used to destroy tumours with a high dose of radiation.
Exposure to radiation
Exposure to radiation can destroy living cell membranes by ionisation, causing the cells to die, or damage DNA which causes mutations that could lead to cancer.
Describe the proces of nuclear fission

Describe the proces of nuclear fusion