Experiment 5 - Emission Spectra & Electronic Structure of Atoms
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
15 Terms
Emission
The process in which an excited atom or electron releases energy as light when it falls from a higher energy level to a lower one.
→ Produces bright spectral lines.
Absorption
The process in which an atom or electron takes in energy (light) and moves from a lower energy level to a higher one.
→ Produces dark lines in a continuous spectrum.
Wavelength (λ)
The distance between two consecutive wave peaks (or troughs) of light.
Usually measured in nanometers (nm)
Shorter wavelength = higher energy
Wavenumber (ṽ)
The number of waves per unit distance, equal to the reciprocal of wavelength.
Units: cm⁻¹
Higher wavenumber = higher energy
When to use the Rydberg equation
Use it ONLY when:
You’re dealing with hydrogen or hydrogen-like atoms (H, He⁺, Li²⁺)
The question involves emission or absorption lines
An electron is moving between energy levels (n values)
🚫 Don’t use it for multi-electron atoms (unless stated hydrogen-like).
atomic emission spectra
When electrons drop to lower energy levels, atoms emit light at specific wavelengths
determine a value for the Rydberg constant
Measure the wavelengths of hydrogen emission lines, then use the Rydberg equation to calculate RRR.
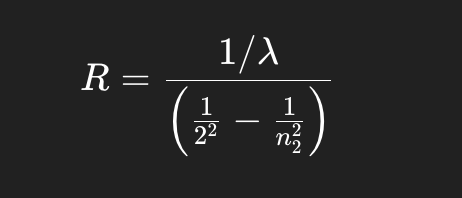
Wavelength → Wavenumber
Convert nm → cm
1 nm=1×10−7 cm
Take the reciprocal
Example:
500 nm →
500×10^−7=5.0×10^−5cm
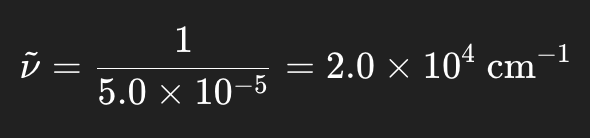
Wavenumber → Wavelength
Take the reciprocal
Convert cm → nm
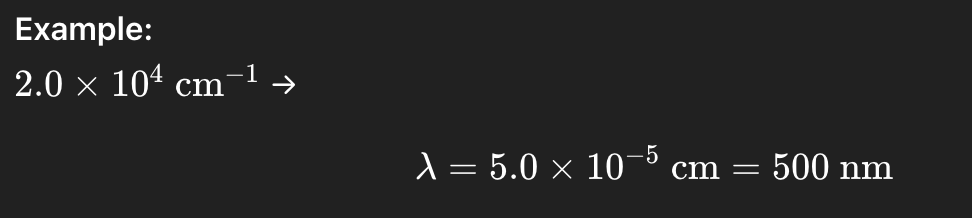
energy level diagram drops reading

Matching to spectra
Each arrow corresponds to one spectral line
Different arrows = different wavelengths
Multiple arrows ending at same level = a series (e.g., Balmer ends at n = 2)
Incandescent (tungsten) spectrum
Produced by a hot solid metal filament
Gives a continuous spectrum (smooth rainbow)
Light comes from thermal energy
No distinct lines
Memory: Hot solid → continuous
Fluorescent lamp spectrum
Produced by excited gas atoms (mainly mercury)
Gives a line spectrum (bright colored lines)
Each line = specific electron transition
Often appears with a weak background
Memory: Excited gas → lines
Emission spectrum
Bright lines on a dark background
Occurs when atoms emit light as electrons fall to lower levels
Characteristic of each element
Memory: Energy out → bright lines
Absorption spectrum
Dark lines on a continuous background
Occurs when atoms absorb specific wavelengths
Same wavelengths as emission lines for that element
Memory: Energy in → missing lines