PE-Medical Technology Laws & Ethics
1/49
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
50 Terms
d. September 13, 2022
The Revised Medical Technologist Code of Ethics was approved by virtue of PRBMT Resolution No. 72 on:
a. March 7, 1997
b. October 2, 2022
c. June 4, 1994
d. September 13, 2022
c. Organizing
Which managerial function involves coordinating resources to achieve plans?
a. Planning
b. Directing
c. Organizing
d. Controlling
d. Contain permanent information that do not require updating
The following statements describe clinical laboratory documents, EXCEPT:
a. Provide written information about policies and procedures
b. Establish formats for recording and reporting information by the use of standardized forms
c. Examples include quality manual, standard operating procedures and bench aids
d. Contain permanent information that do not require updating
a. 1963
The Philippine Association of Medical Technologists was established in:
a. 1963
b. 1964
c. 1969
d. 1973
b. Biostatistics and Epidemiology
The following are professional courses in the new BS Medical Technology curriculum EXCEPT:
a. Molecular Biology and Diagnostics
b. Biostatistics and Epidemiology
c. Medical Technology Laws and Bioethics
d. Mycology and Virology
b. Autoverification
Which of the following refers to a process where computer-based algorithms automatically perform actions on a defined subset of laboratory results without the need for manual intervention?
a. Barcoding
b. Autoverification
c. Interfacing
d. Connectivity
c. RA 11223 (Universal Health Care Act)
Which law mandates the incorporation of educational outcomes focusing on primary care in the education programs, scope of licensure examinations, CPD programs for health professionals, and certification programs for healthcare workers?
a. RA 10173
b. RA 10912
c. RA 11223
d. RA 11332
c. Elimination
Which is the most effective measure in the hierarchy of controls?
a. PPE
b. Substitution
c. Elimination
d. Engineering controls
b. Foreign reciprocity
A foreign medical technologist whose government allows Filipino medical technologists to practice in their country may be allowed to practice his profession in the Philippines. This is called:
a. Consultancy
b. Foreign reciprocity
c. Treaty of professions
d. Unconditional recognition
b. 3 times
A refresher course should be taken by applicants who shall have failed the Board Examination _____.
a. 2 times
b. 3 times
c. 4 times
d. 5 times
a. professional skills and knowledge
According to the revised code of ethics, registered medical technologists shall commit themselves to continuously improve their:
a. professional skills and knowledge
b. reputation and dignity
c. technical competence
d. communication and interpersonal skills
d. a pathologist as chairman and two medical technologists as members
As provided under RA 5527, the Board of Medical Technology shall be composed of:
a. a pathologist as chairman and three medical technologists as members
b. a pathologist as chairman and a medical technologist as member
c. a medical technologist as chairman, a pathologist and another med tech as members
d. a pathologist as chairman and two medical technologists as members
b. 2 and 4
Whose signatures are seen in the certificate of registration of a professional?
1. Professional Regulatory Board secretary
2. Chairman and members of the Board
3. Associate commissioners of PRC
4. Chairperson of PRC
a. 1 and 3
b. 2 and 4
c. 1, 2, and 3
d. 1, 2, 3, and 4
d. Approve schools of medical technology (CHED)
The following are functions of the Board of Medical Technology, EXCEPT:
a. Conduct licensure examinations
b. Administer professional oath
c. Conduct administrative investigation
d. Approve schools of medical technology
a. CHED
Which government agency approves clinical laboratories for accreditation as training institutions?
a. CHED
b. DOH
c. PRC
d. BRL
c. It cannot be used without diluting it with water.
Which of the following statements is inaccurate given the following information on an NFPA hazard warning?
Blue Diamond: 3
Red Diamond: 4
Yellow Diamond: 2
White Diamond: W
a. It poses extreme danger to the health of the worker.
b. Its flashpoint is below 73° Fahrenheit.
c. It cannot be used without diluting it with water.
d. It may undergo violent chemical changes.
c. 3 out of 3
To revoke a license or certificate of registration, the required number of votes by the members of the Board is:
a. 1 out of 3
b. 2 out of 3
c. 3 out of 3
d. B or C
d. June 21, 1969
The Philippine Medical Technology Act was approved on:
a. June 28, 1969
b. August 31, 1969
c. June 11, 1969
d. June 21, 1969
c. Mature Minor Doctrine
Which legal principle recognizes the capacity of some minors to consent independently to medical procedures, if they have been assessed to understand the nature of procedures and their consequences to make a decision on their own?
a. Evolving Capacities of a Child Principle
b. Principle of Double Effect
c. Mature Minor Doctrine
d. Ethical Competence
b. 3
The renewal of professional identification card is once every how many years on or before the birthday of the professional?
a. 1
b. 3
c. 4
d. 5
b. Motors, switches, computers (Curyente)
Class C fire may involve which of the following?
a. Wood, plastic, cloth
b. Motors, switches, computers
c. Combustible metals
d. Paints, gasoline, LPG
d. Clinical Microscopy (and Histopathology, MTLE, & Lab Mgmt. and Research)
The following licensure examination subjects each have a weight of 20% EXCEPT:
a. Clinical Chemistry
b. Hematology
c. Microbiology and Parasitology
d. Clinical Microscopy
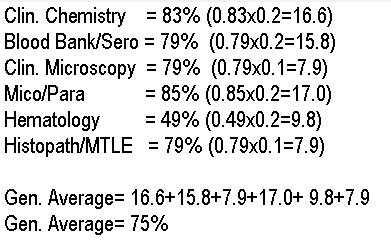
d. 75%
What is the general average of an examinee who gets the following ratings in the licensure
examination?
Clin. Chemistry = 83%
Blood Bank/Sero = 79%
Clin. Microscopy = 79%
Mico/Para = 85%
Hematology = 49%
Histopath/MTL = 79%
a. 75.3%
b. 74.5%
c. 74.9%
d. 75%
d. 21 years of age (minimum age for issuance of COR)
The following are qualifications for examination as stipulated in RA 5527 EXCEPT:
a. BSMT graduate
b. in good health
c. of good moral character
d. 21 years of age
d. Board of Medical Technology
Before entering the practice of medical technology, licensure passers are required to take a professional oath before the:
a. Council of Medical Technology
b. PRC Commissioner
c. President of the Philippines
d. Board of Medical Technology
d. Identifying the causes of problems in the laboratory and the solutions to those problems
Which of the following correctly describes quality improvement as part of total quality management in the laboratory?
a. Monitoring laboratory performance such as turnaround times, specimen and patient ID procedures, and test usage
b. Performing statistical control procedures
c. Ensuring that the laboratory’s policies, procedure, and practices are followed
d. Identifying the causes of problems in the laboratory and the solutions to those problems
a. RA 4688 (Clinical Laboratory Act)
An act regulating the operation and maintenance of clinical laboratories and requiring their registration:
a. RA 4688
b. RA 1517
c. RA 7719
d. RA 6138
c. RA 7719 (National Blood Services Act)
An act promoting voluntary blood donation and providing for the phasing out of commercial blood banks:
a. RA 6138
b. RA 1517
c. RA 7719
d. RA 4688
b. RA 11166
The Philippine HIV/AIDS Policy Act of 2018 is also known as:
a. RA 8504
b. RA 11166
c. RA 16611
d. RA 10586
c. Inconclusive
How should HIV test result be reported if there is any inconsistency with screening and/or rHIVda RDT results?
a. Positive
b. Negative
c. Inconclusive
d. Non-reactive
b. prescribe the qualifications of medical technologists as to special fields of the profession
The following functions and duties of the Board were repealed by PD 1534 except:
a. determine the adequacy of staff of laboratories and blood banks before they could be licensed
b. prescribe the qualifications of medical technologists as to special fields of the profession
c. classify the technical staff according to seniority
d. none of the above
d. 13
Primary: 8 (w/o Micro), 9 (government facilities)
Secondary: 12
Tertiary: 12 (w/o histopathology), 13 (hospital-based)
What is the prescribed minimum number of personnel for tertiary hospital-based clinical laboratory?
a. 8
b. 9
c. 12
d. 13
c. name, address, citizenship, date of registration
What information may be found in the roster of medical technologists?
a. license number, name, age, address
b. profession, name, birthdate, rating
c. name, address, citizenship, date of registration
d. name, gender, address, date of issuance of COR
a. function
Whether a laboratory is clinical, anatomical, or molecular is a classification by:
a. function
b. ownership
c. service capability
d. institutional character
a. East Avenue Medical Center
Which is the national reference laboratory for environmental and occupational health, toxicology, micronutrient assay?
a. East Avenue Medical Center
b. Research Institute for Tropical Medicine
c. Lung Center of the Philippines
d. San Lazaro Hospital
d. 100 km
A mobile clinical laboratory is allowed to operate at a maximum of ________ radius, from the DOH-licensed CL.
a. 10 km
b. 50 km
c. 100 m
d. 100 km
b. proficiency testing (NEQAS/EQAP)
A program in which samples are periodically sent to members of a group of laboratories for analysis is called:
a. quality control
b. proficiency testing
c. accreditation
d. benchmarking
a. R.A. 7722 (Higher Education Act)
Which law created the Commission on Higher Education?
a. R.A. 7722
b. R.A. 7719
c. R.A. 8504
d. R.A. 9288
a. CMO No. 13 series of 2017
The latest CHED memorandum order providing for the policies, standards, and guidelines for the Bachelor of Science in Medical Technology/ Medical Laboratory Science program is:
a. CMO No. 13 series of 2017
b. CMO No. 06 series of 2018
c. CMO No. 14 series of 2006
d. CMO No. 52 series of 2016
c. Newborn Screening Act
Republic Act 9288 is otherwise known as:
a. Dangerous Drugs Act
b. PRC Modernization Act
c. Newborn Screening Act
d. Water Analysis Law
d. homocystinuria
Newborn Screening Panel of Disorders:
-Phenylketonuria
-G6PD Deficiency
-Galactosemia
-Congenital Hypothyroidism
-Congenital Adrenal Hyperplasia
-MSUD (Maple Syrup Urine Disease)
The following are included in the six basic disorders tested for as part of newborn screening except:
a. phenylketonuria
b. galactosemia
c. G6PD deficiency
d. homocystinuria
d. EDTA whole blood (for confirmatory test)
(Dried blood spot: for screening test)
What is the required specimen for confirming a positive G-6-PD deficiency?
a. dried blood spot
b. urine
c. serum
d. EDTA whole blood
a. 28
How many disorders are covered by the expanded newborn screening program?
a. 28
b. 26
c. 20
d. 8
c. name and place of residence
In the first line of the “Panunumpa ng Propesyonal”, the professional states his
a. name and profession
b. name and school
c. name and place of residence
d. name and citizenship
d. removal from the roster
If a registered medical technologist will not renew his license in 5 years, what would be the consequence?
a. fine of P2000.00
b. perpetual disqualification
c. revocation of COR
d. removal from the roster
b. RA 8981 (PRC Modernization Act of 2000)
Which law mandated the full computerization of the licensure examinations?
a. RA 7722
b. RA 8981
c. RA 9288
d. RA 9165
d. President of the Philippines
The chairman and members of the Board of Medical Technology are appointed by the:
a. CHED Commissioner
b. PRC Chairman
c. DOH Secretary
d. President of the Philippines
b. July 21, 2016
The Continuing Professional Development Act lapsed into law on:
a. June 21, 2006
b. July 21, 2016
c. August 22, 2007
d. May 2, 2008
b. HFSRB
Which agency is mandated to establish the standards for regulation of hospitals, clinics, and other health facilities and to enforce such standards which shall be the basis for the issuance of license to operate?
a. NRL
b. HFSRB
c. BRL
d. RITM
b. recommend members of the various boards for appointment to the president of the Philippines
Which of the following is one of the functions of PRC?
a. appoint the members of the various professional regulatory boards
b. recommend members of the various boards for appointment to the president of the Philippines
c. remove from office any member of the professional regulatory boards
d. approve the accreditation of schools of medical technology