bio20 ch2 - cycles of matter
1/38
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
39 Terms
universal solvent
property of water
- AKA high solvency, due to polarity. water has a slight POSITIVE charge at one end, and a slight NEGATIVE charge at the other end.
- allows for hydrogen bonds. together enables water to dissolve a wide variety of substances - both molecular and ionic = carries both nutrients & toxins
relatively high melting, boiling points
property of water
- the more H-bonds there are, the more energy it takes to breath them
- water remains liquid over high ranges of temperatures
high specific heat capacity
property of water
- high energy needed to change temp. of water even 1C = long time to heat up or cool down.
- allows organisms with lots of water in their tissues to maintain a fairly constant temp.
- large bodies of water have an effect on air temperatures of nearby land
cohesion/adhesion
property of water
- cohesion : attraction of water molecules to other WATER molecules. this creates surface tension = the tendency of a liquid's surface to resist rupture when placed under tension or stress (think droplets on a coin!)
- adhesion : attraction of water molecules to OTHER substances
density of ice
property of water
- H-bonds allow frozen water to be LESS dense than liquid water = ice on top of water = habitats etc.
- also leads to cycling of water, nutrients
biogeochemical cycles
a complex, cyclic transfer of nutrients from organism to the environment back to an organism. driven by solar energy, involves photosynthetic/chemosynthetic organisms
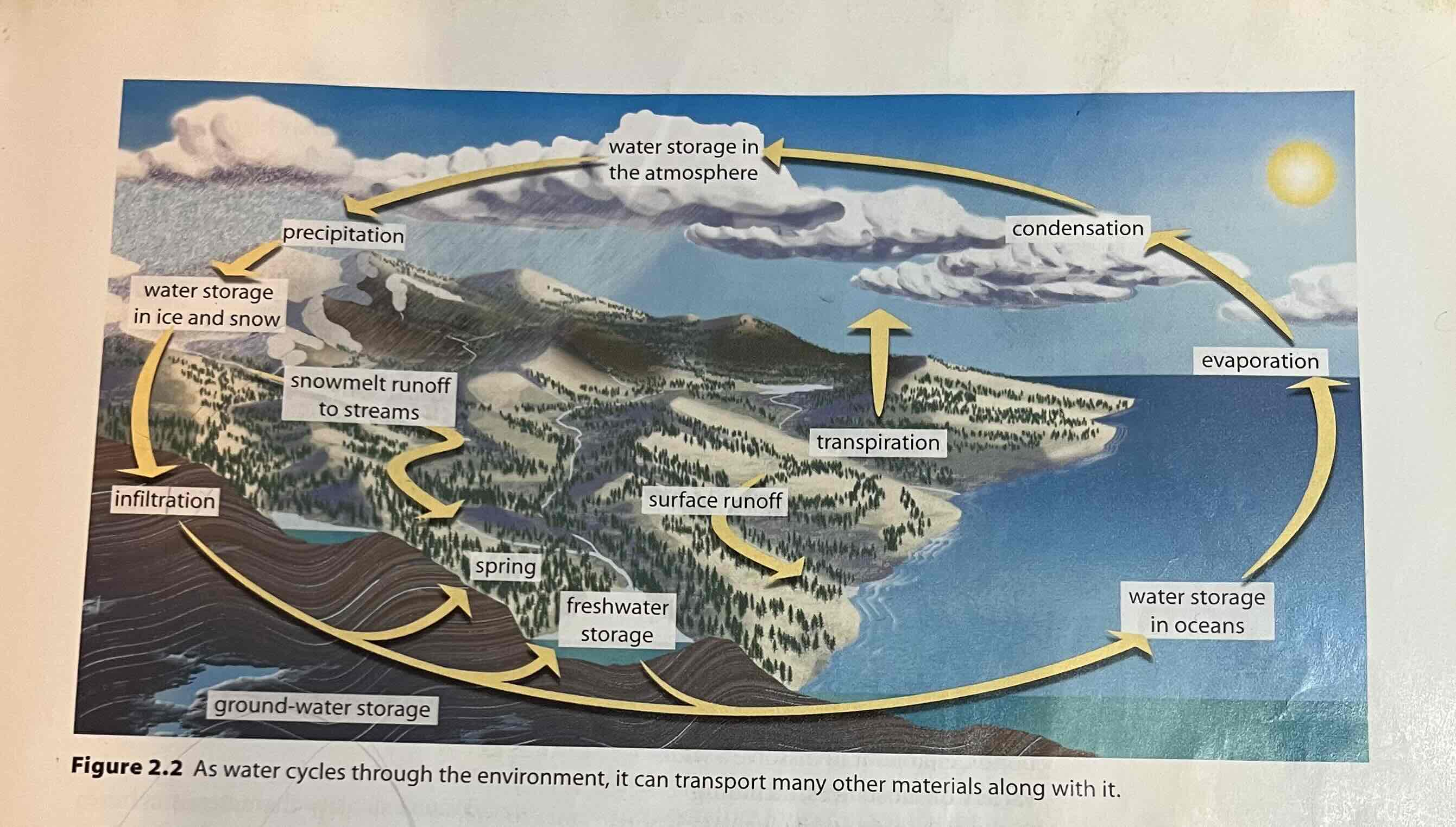
hydrologic cycle
The cycle through which water in the hydrosphere moves; includes such processes as evaporation, precipitation, and surface and groundwater runoff
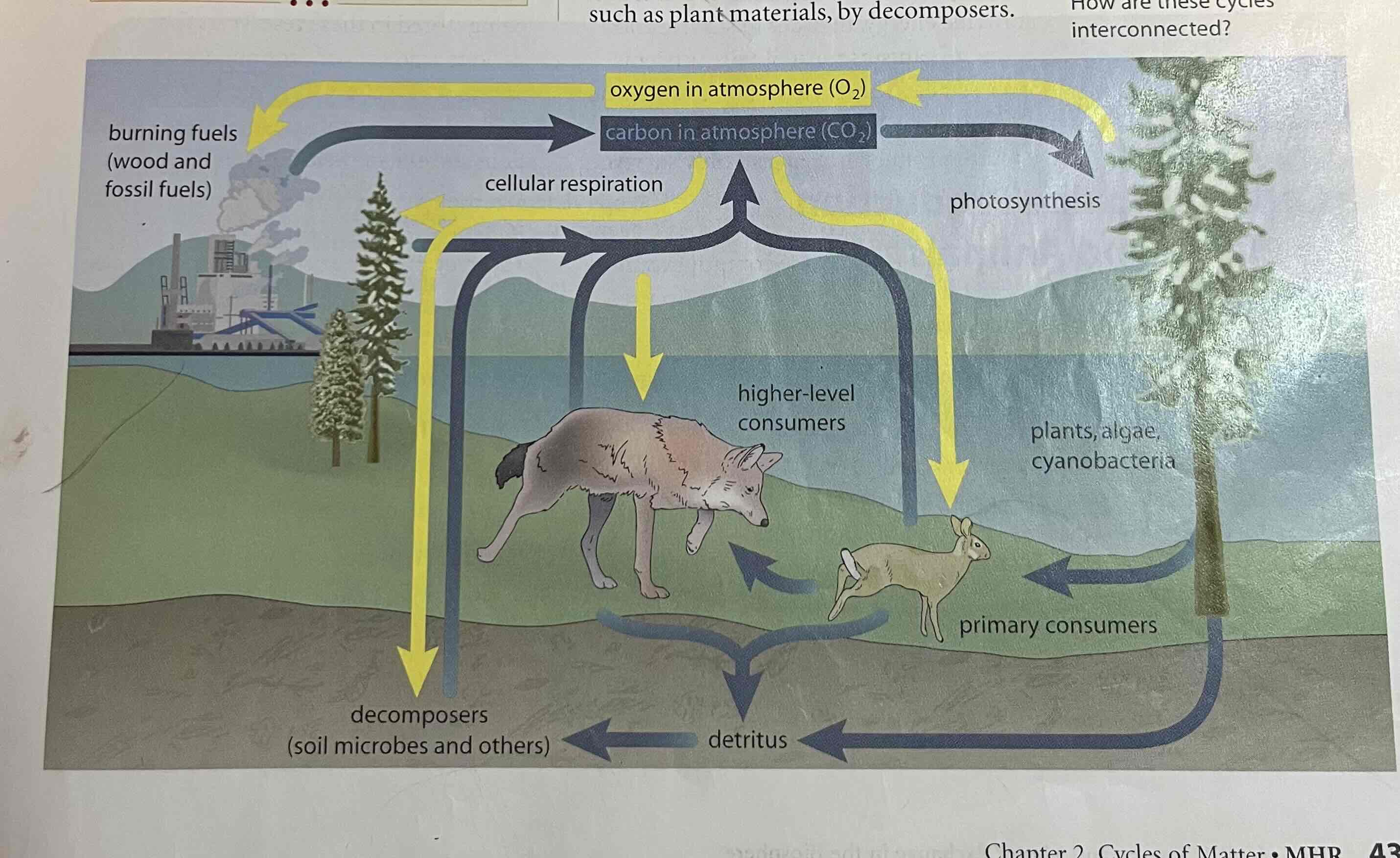
oxygen cycling
vey complex, inter-related to carbon cycling due to cellular respiration, photosynthesis, and decomposition.
- also involves storage of oxygen in lithosphere. an be converted to O3 (ozone) in the atmosphere and to water in the hydrosphere
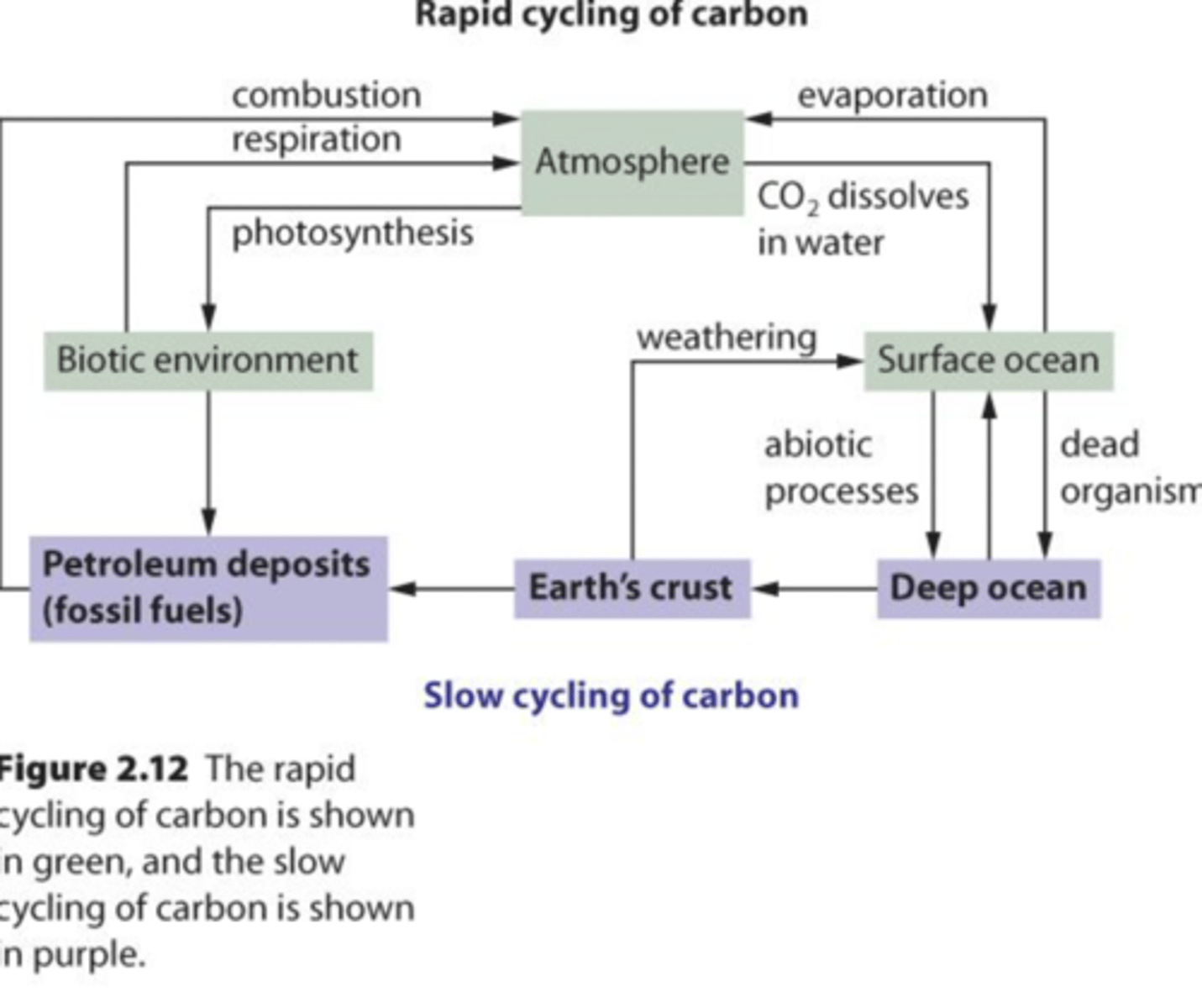
rapid cycling
- substances cycle between nutrient reservoirs quickly.
ex. carbon moving through food chain to environment
slow cycling
- substances accumulate in reservoir and are unavailable to organisms.
ex. fossil fuels take millions of years to form
carbon cycling
- Plants, algae, and bacteria take in atmospheric carbon dioxide and use it to perform photosynthesis, bringing carbon into the living world. Animals then obtain their carbon from these photosynthesizing organisms.
- The carbon cycle is completed when carbon moves back into the atmosphere as carbon dioxide, produced through the respiration of living things and the decomposition of dead organisms.
carbon-oxygen cycle
the process by which carbon and oxygen cycle among plants, people and animals, and the environment
phosphorus cycle
The movement of phosphorus atoms from rocks through the biosphere and hydrosphere and back to rocks.
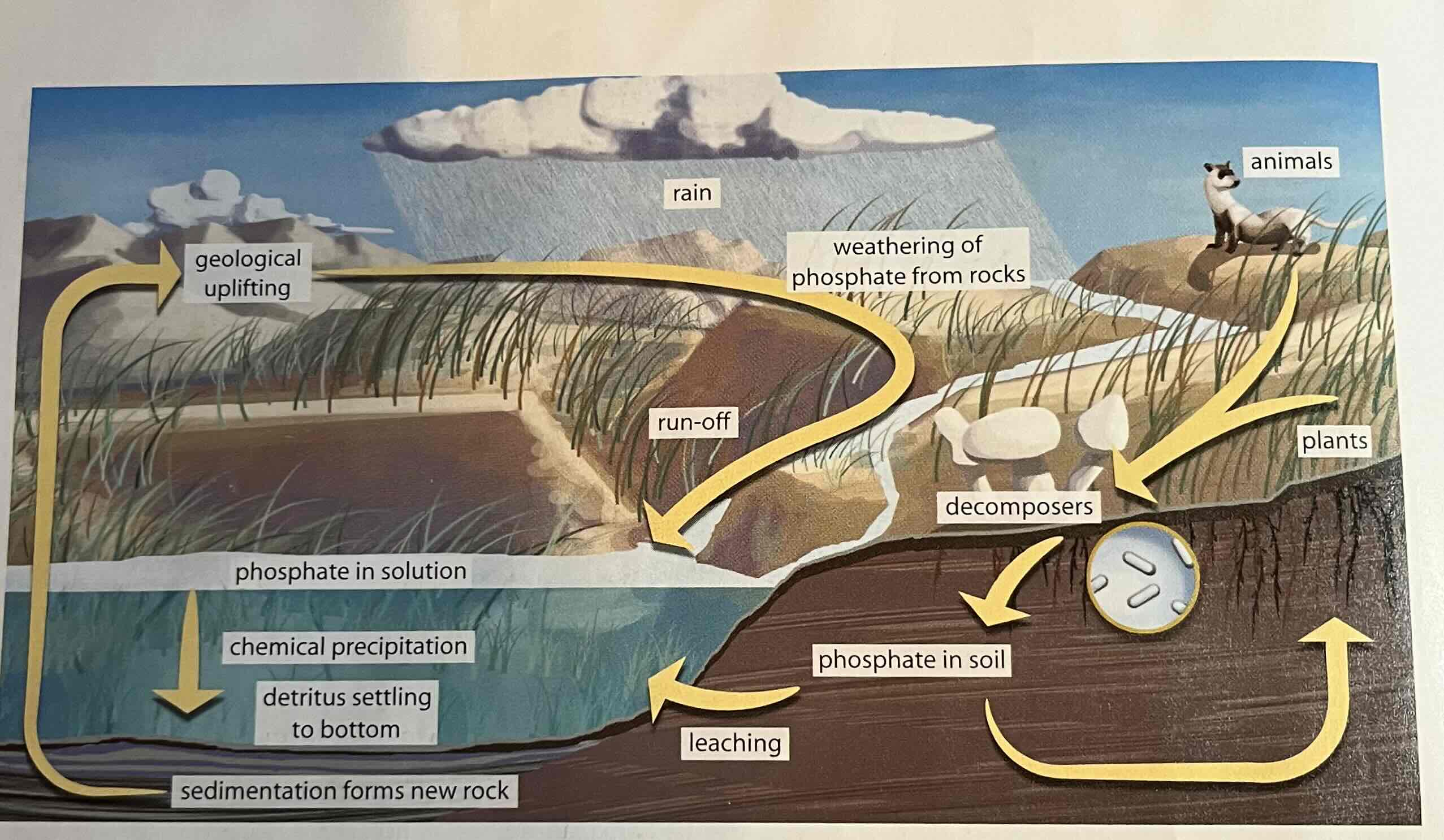
long cycle
abiotic = rocks in Earth's crust, millions of years
short cycle
biotic = phosphates (PO₄³⁻); soluble in water
system
object(s) or selected areas involved in the transfer of energy and/or matter
open system
matter and energy are exchanged with surroundings
closed system
cannot exchange matter with surroundings, only energy
isolated system
cannot exchange matter nor energy
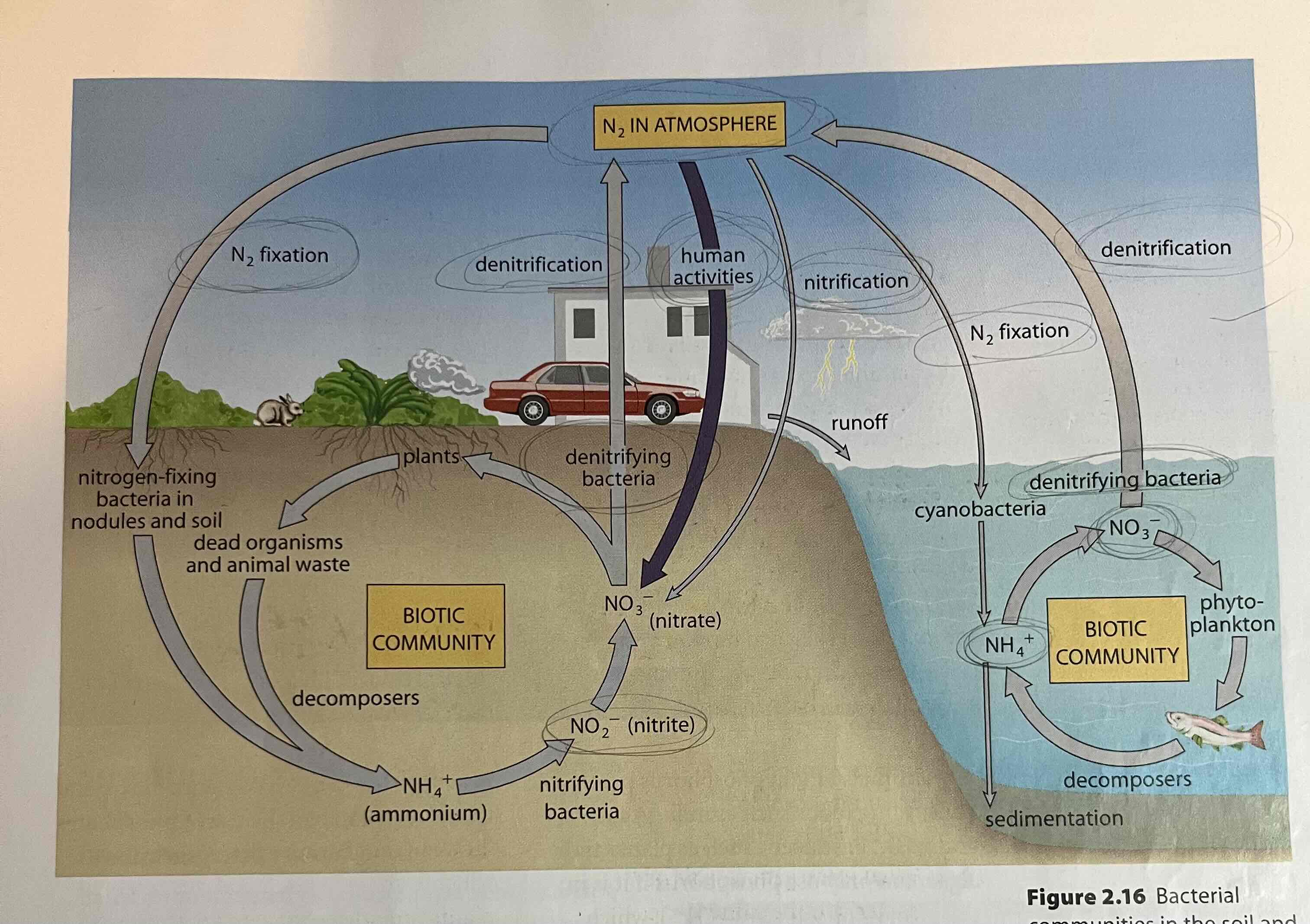
nitrogen fixation
conversion of atmospheric nitrogen into biotic nitrogen
atmospheric nitrogen fixation
- lightning will convert small amounts of N₂ into NO₃⁻ (nitrate) or ammonium (NH₄⁺)
bacterial nitrogen fixation
- N fixing bacteria (either in soil, or on roots) provides nitrates or ammonia for plants
ammonification
(decay) breakdown of organic matter by decomposers - produces ammonium (NH₄⁺)
- fungi and bacteria decompose organic matter to produce ammonia or ammonium
- ammonium can be used by select plants to make amino acids
nitrification
nitrifying bacteria in the soil convert NH₄ into nitrite (NO₂) and then nitrate (NO₃) - plants use as a nitrogen source
- nitrates can be used by all plants to make amino acids
assimilation
(protein synthesis, biosynthesis)
- plants incorporate nitrates, ammonium into organic compounds.
- animals consume other organisms and reorganize into its organic compounds
denitrification
denitrifying bacteria complete the cycle by converting nitrite or nitrate back into nitrogen gas
in the soil
NO₃⁻ nitrates -> taken up by plants
NO₂⁻ nitrites -> need to be converted into nitrates for plant take up
NH₃ ammonia -> can be taken up by some plants, but most converted to nitrates before majority of plants can use
in the air
N₂ (nitrogen gas) -> stable, unreactive, not useable for living things
in living organisms
protein -> component in plants and animal tissue (amino acids)
nucleic acids -> component of DNA & RNA (hereditary material)
eutrophication
A process by which nutrients, particularly phosphorus and nitrogen, become highly concentrated in a body of water, leading to increased growth of organisms such as algae or cyanobacteria.
algae blooms
a rapid increase in the population of algae (informal term for a large, diverse group of photosynthetic organisms) in an aquatic system. can occur seasonally in response to the turnover of nutrient-rich waters in warmer temperatures. they often result in "dead zones" = regions of lakes or oceans in which aquatic life as suffocated
dead zones
regions of lakes or oceans in which aquatic life as suffocated
nitrogen cycle
The transfer of nitrogen from the atmosphere to the soil, to living organisms, and back to the atmosphere
productivity
the rate at which an ecosystem‘s producers can capture and store energy or the rate organisms can produce new biomass
productivity rates are often expressed as energy or biomass
the amount of sunlight an area receives determines its productivity
rate of productivity
depends on…
number of producers in an ecosystem
amount of heat and light available
amount of rainfall
4 steps that lead to eutrophication
1) nutrients enrichment due to runoff from agricultural fields
2) rapid growth of algae and other planktons resulting in agal blooms
3) dissolved O2 used up by decomposers
4) aquatic species die due to lack of O2
sulfur cycle
volcanoes, weathering, and mining coal can release sulfur into the atmosphere as sulfur dioxide (SO2)
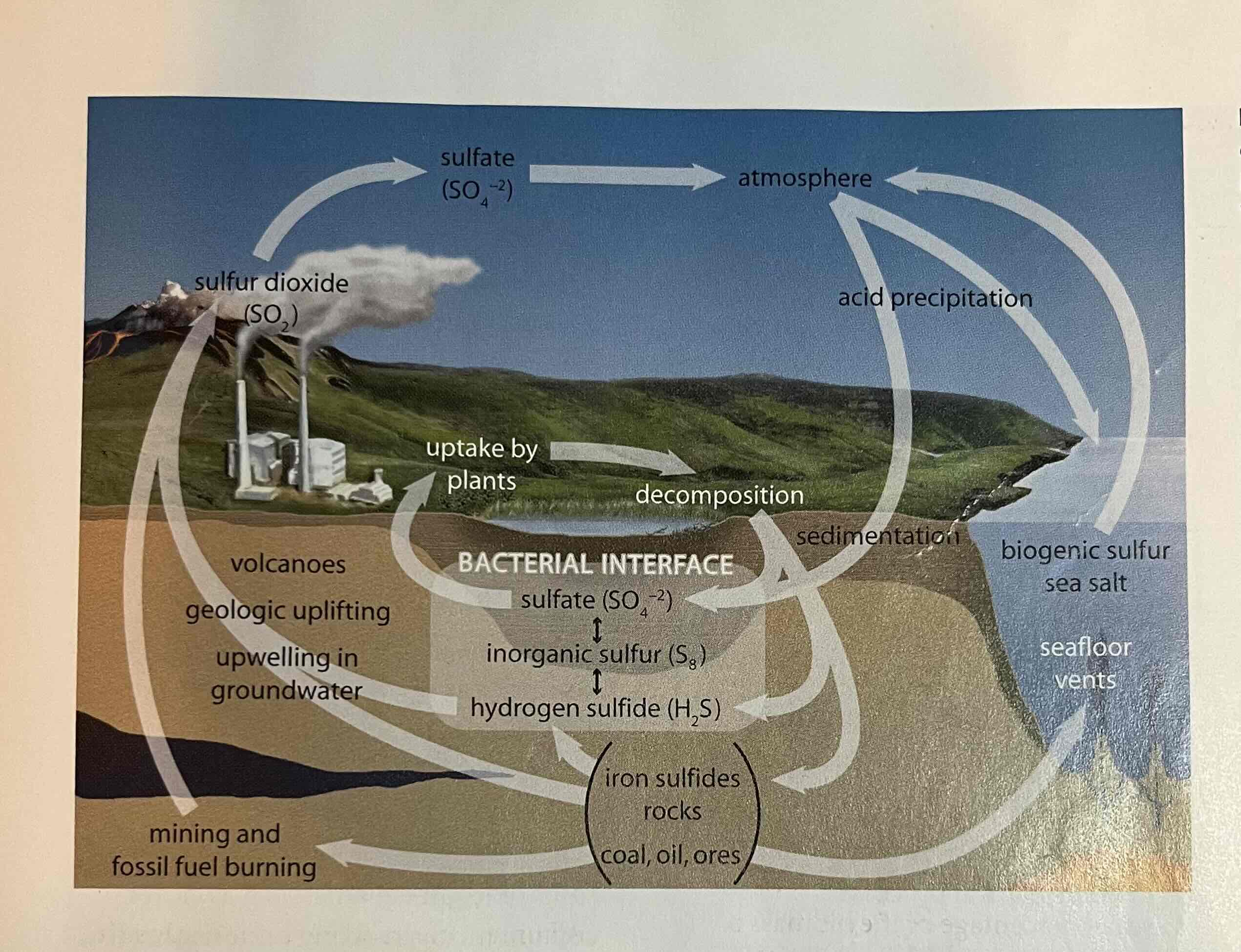
acid deposition
SO2 in atmosphere reacts with O2 and H2O to form H2SO3 (sulfurous acid) and H2SO4 (sulfuric acid)
this results in acid deposition (acid rain) - returns sulfur to oceans and soils.
can damage plants, acidify lakes and leach nutrients from the soil
human activities such as fossil fuel burning can cause release of excess sulfur
gaia hypothesis
the Earth is self-regulating maintaining its environmental conditions within certain limits. To maintain internal balance, the biosphere needs a constant input of energy and cycling of nutrients
James Lovelock proposed that the biosphere acts like an organism that regulates itself (homeostasis on a global level)