Carboxylic Acids and Derivatives Reactions and Stuff
1/36
Earn XP
Description and Tags
LORD i pray this helps me!
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
37 Terms
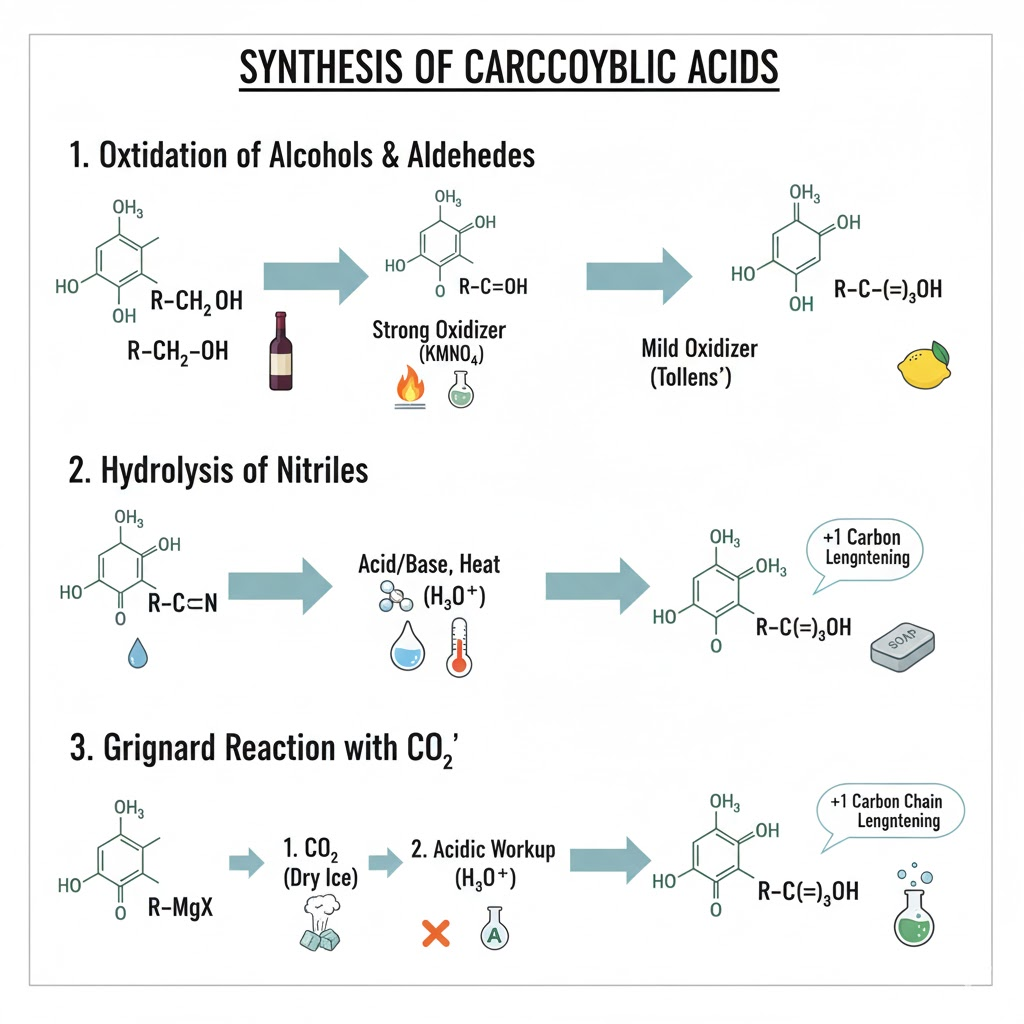
How do you make carboxylic acids
Take a primary alcohol/aldehyde and Jones it (H2CrO4 H2O)
Add CO2 to grinard reagent, then acid workup
Take a nitrile CtriplebondN, hydrolysis with acid catalyst, so H2SO4, H2O, Heat to create carboxylic acid and amine. (NH3 if base, NH4+ if acid)
Reduction of Carboxylic Aicd
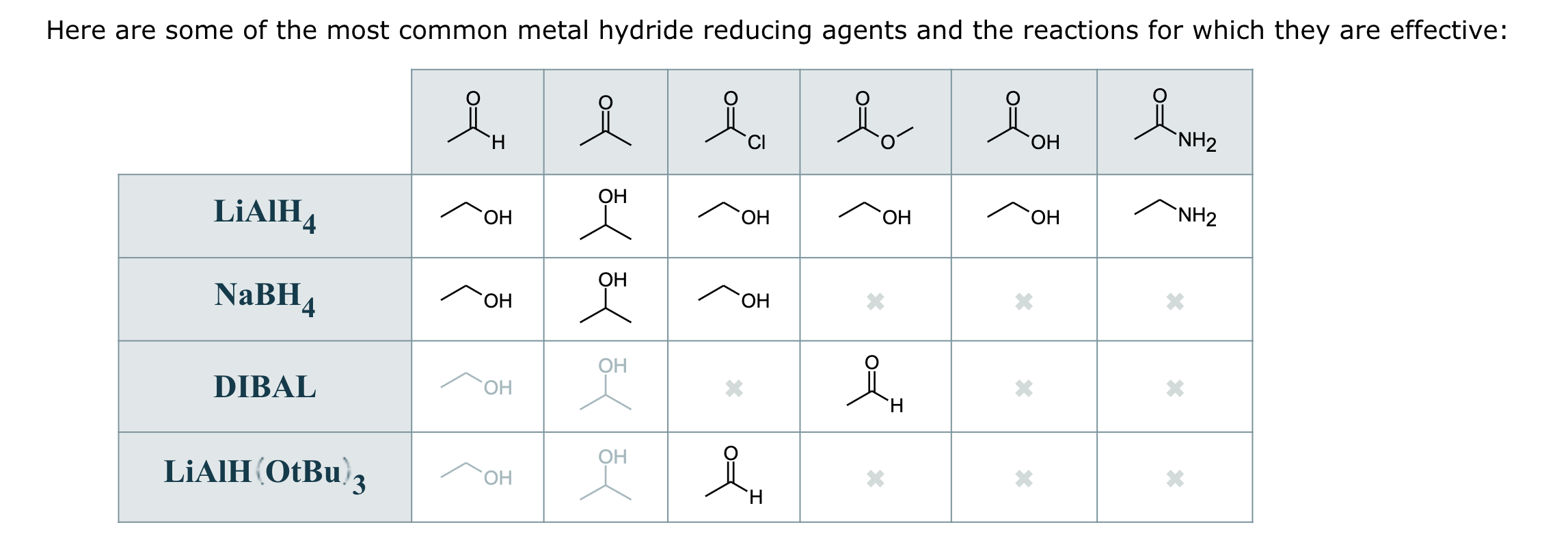
Only LAH or 1. LiAlH4, Et2O 2. HCl, H20 to primary alcohol
What happens when you react a carboxylic acid with an alcohol?
With an acid catalyst!! FISCHER ESTERIFICATION PADPED
How can you make a carboxylic acid into a ester?
Fischer esterification, turning a carboxylic acid into an ester with an alcohol and an acid catalyst (NOT base)
Esterification, methyl esters
Uses CH2N2, Et2O, -78 C
Diazomethane, where carbanion in Ch2N2 attacks OH, taking H, then giving CH3 back, N2 leaves as leaving groups
How do you make an acid chloride?
SOCl2
(will also create HCl, SO2)
Decarboxylation
Only happens with Carbonyl group 2 positions away in Beta Position
Heat (Delta), -CO2
Twist Carboxylic group, make enolate ion and take away CO2
HYDROLYSIS OF DERIVATIVES
Makes Carboxylic Acid and maybe byproducts
Very reactive Carboxylic Acid Derivative, what are they and what do they need?
Acyl Chloride and Acid Anhydride
Only neutral water
Anhydride sometimes acid catalyst (PADPED)
Less Reactive Carboxylic Acid Derivative
Ester
Amide
Nitrile
These can hydrolyze with Take home conditions or to the doorstep conditions
Take Home Conditions
H2O, Heat, H2SO4 (Product: Carboxylic Acid)
DoorStep conditions
NaOH (Aq), Heat
Product = Carboxylate salt
Nitrile hydrolysis ACID catalyzed mechanism
Complete from Nitrile to Carboxylic acid and NH4+ is PADPADPED
Nitrile Hydrolysis BASE catalyzed mechanism
You have to attack OH twice, Make the double O negative so that NH2 finally leaves as a leaving group. NH2 becomes NH3
Anhydrides + H2O
2 Carboxylic Acids
Esters + H2O, H2SO4 (cat.)—>
1 carboxylic acid, 1 alcohol (Reversal of FIscher esterification)
Ester + 1. NaOH, H20 2. HCl, H20
special stoichiometry
1 Carboxylic Acid, 1 Alcohol
YOU NEED A WHOLE STOICHIOMETRIC BASE (1:1 ester to base)
Amides + HCl, H20, heat
Stoichiometric equivalence of HCl needed, AND HEAT
Produces Carboxylic Acids and NH4Cl
PADPED
Amide + NaOH, H20, Heat
Carboxylate Ion Na+ and Amine H2N—PH
need Acid work up, HCl, H2O so product: carboxylic acid and NH3 + —-PH
two negative O’s in mechanism
Nitriles + H2O, HCl, Heat
Amide then 2 eqiuvalence, is Carboxylic acid.
How to write and make a carboxylic acid through C=N starting with CHxBr
KCN, DMSO
NAOH, H2O, HEAT
HCL, H20
Acid Chloride + ALCOHOL, what are the solvents?
You NEED pyridine or NEt3
Acid Chloride + CH3OH ——(pyridine)—→ creates what, what is the mechnaism first step??
No catalyst required
Amine Base is added as HCL sponge.
Follows an Addition, Elimination pattern AND STARTS W PYRIDINE BC N IS MORE NUCLEOPHILIC THAN O (Less EN)
Pyridine attacks acid chloride first
Then Cl leaves
then OH attacks
Pyridine leaves, creating ester product.
Anhydrides + ALCOHOL + Catalyst
Creates a Carboxylic acid and an ester , which carboxylic acid can be turned into another ester w alcohol and h2so4 cat.
Transesterification
Ester and Alcohol make a new ester and alcohol (replace OR group with R group in OH) (Basically r groups of alcohol and ester switch!!)
NEEDS ACID CATALYST AND HEAT
Amides + Alcohol
NO REACTION
Acid Chloride + Ammonia, 1, 2 solvent
Pyridine, CH2CL2
Acid Chloride + NH3 (1 or 2)—→
Creates an Amide and pyridineNH+Cl (shortcut NR2 connected to carbonyl C, 1 H in Pyridine N)
NH3 Sp3 more powerful attack Carbonyl C, then deprotonate NH3
you need pyridine and ch2cl2 as solvent
Anhydrides + Ammonia, 1, of 2 Amine
Pyridine, Ch2Cl2 Solbvent
Products: Amide, Carboxylate Ion and NH4+Pyridine
Thing to remember, carboxylate ion is the LEAVING GROUP
ESTER + AMINE
No rxn
Amides and amines
No rxn
How to make asymmetric Anhydrides
1. React Acid Chlorides with Carboxylate Anions
This will create Anhydride and KCl as byproduct
Solvent: Pyridine or NEt3
Have carboxylate anion attack the carbonyl c, getting rid of Cl. Customize R groups for asymmetric.
How to make symmetric anhydrides
Take 2 Carboxylic Acids, react with P2O5, make sure to have all carbons still there, just replace OH w O and connect carbon chains
Acid Chloride and Grignard or Organolithium
Add twice to form alcohol product (tertiary)
1 eqiv will form ketone intermediate but cannot isolate
assume 2 equiv
CH3Li, (2 equiv), et2O
Hcl, H20 workup
Attack Eliminate mechanism, then protonate
Grignard and Organolithium and Nitriles
Gives Ketones, NH4+Cl
CH3Li, et20
HCL, H20.
Form A nitrile, Protonate Nitrile, then Imine hydrolysis(hemiaminal thenn OH)
Carboxylic Acids and Organolithium Reagents
No grinyard, just organolithium
Ch3Li (2 eq), Et20
Hcl, H20
Creates Ketones!! (And a carbonyl hydrate)
forms that di oxygen ion complex
Gilman and blank
ONLY ACID CHLORIDES TO GIVE KETONES!!!
(CH3)2CuLi, Et20
HCL, H20