BIOMATERIALS EXAM 1 - INTRODUCTION TO MATERIALS IN BIOMEDICAL APPLICATIONS
1/23
Earn XP
Description and Tags
lectures 1-2
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
24 Terms
what is a biomaterial?
a material intended to interface with biological systems to evaluate, treat, augment, or replace any tissue, organ, or function of the body
stages of biomedical development: (3)
inert, guide biological function, completely replace
example of inert: (2)
glass eye, teeth
example of guide: (2)
hearing aids, contacts
example of replace: (2)
skin graft, artificial tendon
biological responses to biomaterials: (6)
immune response, blood clotting, infection, tumor, calcification, fibrosis
are biological responses desirable or undesirable?
both, depends on the application
ex: calcification in a bone implant vs contact
types of biomaterials
organic (containing carbon) - polymers: natural, synthetic
inorganic (no carbon) - metals, ceramics
metals:
highly mobile electrons
conduct electricity, easily form complex shapes
suitable for orthopedic and dental implants, stents
also includes alloys, combination of elements
usually used for strength
ceramics:
non-direction ionic bonds between electron-donating and electron-accepting elements
crystalline vs amorphous ceramics
hard and resistant to degradation
brittle, only employed for smaller loads
like frozen chewing gum
very strong, sometimes more than metal, but brittle
are ionic or covalent bonds stronger?
ionic
polymers:
long chains held together by directional covalent bonds
large range of physical and chemical properties
can form composite materials to improve bulk or surface properties where only one is a polymer
may not be high in strength
natural polymers:
can be derived from sources within the body (collagen, fibrin, hyaluronic acid) or outside the body (chitosan, alginate)
most common in the body is collagen - matrix that holds everything together in the body
have chemical compositions similar to tissues
large quantities of these materials, mechanical properties low
already in nature, humans or animals, so body most likely will not react poorly
synthetic polymers:
can be mass produced and sterilized without affecting its properties
physical, chemical, and mechanical properties can be tailored
reactivity in vivo is not as good as natural
so you can make large amounts but may not react well
degradative properties of biomaterials:
can be both good or bad depending on the application; environment and properties are factors
surface properties of biomaterials:
can make or break assimilation of a biomaterial within an environment (due to protein interaction)
surface is a few atomic layers on the exterior of the object (can be different from the rest of the object - bulk)
can be achieved by using special processing methods that only affect outermost layers
only a couple of nm thick, cells will initially react with it
includes physical and chemical characteristics
physical surface properties:
don’t think of only cells, applies to other applications
toilet paper holder with different surface material - antibacterial
why not just change the entire material?
you would change the bulk properties that you still want, ex don’t need the inside of the toilet paper holder to be antibacterial too
chemical surface properties:
changing the chemical properties of the outermost layers
changes how to enviroment responds, how cells/proteins may react
easy experiment to determine hydrophobicity/hydrophilicity
drop of water on sample, the angle that forms with the surface give the relative hydrophobicity/hydrophilicity
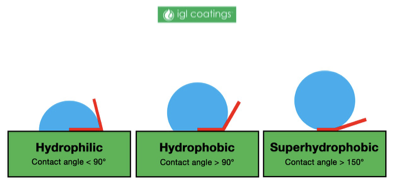
would a hydrophobic or hydrophilic polymer be a more appropriate choice for a contact lens application and why? would a melting temperature above or below 37°C be more appropriate for this application and why?
A hydrophilic polymer would be more appropriate to interface with the aqueous environment of the eye. Additionally, a melting temperature above 37°C would be more appropriate, as one would not want the polymer to be molten at body temperature (37°C) in this application.
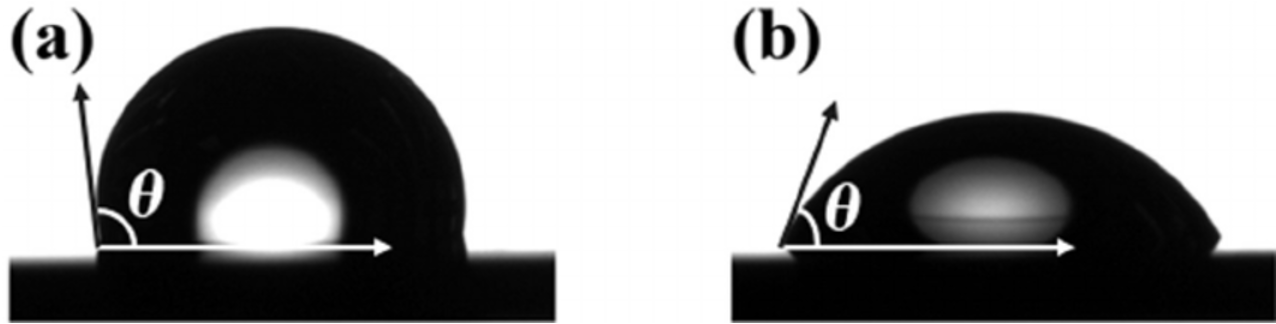
which material is more hydrophilic and why?
b - the angle is smaller
bulk properties:
initially surface properties determine initial response, but overtime bulk properties determine long term impact
include mechanical, physical, and chemical properties
characterization techniques:
techniques used to understand physical, chemical, and mechanical properties of biomaterials
quantitative procedures produce a numerical measure of a property (in absolute or relative units)
qualitative experiments give a general overview of the property of interest without a numerical value
ex: observing a material under a microscope is qualitative while spectroscopy will give us the exact concentration of a material