Sulphuric acid chemical formula
1/17
Earn XP
Description and Tags
H2SO4
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
18 Terms
Hydrochloride acid chemical formula
HCL
Nitric acid chemical formula
HNO3
Ethanoic acid chemical formula
Ch3CO2H
Properties of acids
They have a ph of less than 7
They react with most metal to make hydrogen gas
They react with with carbonates to make CO2
They turn blue litmus paper red
They turn universal indicator red/yellow
MASH
Metal + acid = salt + hydrogen
BASW (British airways Star Wars)
Base + acid = ammonium salt
ACSWCD (a car sinks with cookie dough)
Acid+ carbonate= salt + water + carbon dioxide
Alkali definition
A base that can dissolve in water or a substance that can release hydroxide ions (OH-)in an aqueous solution
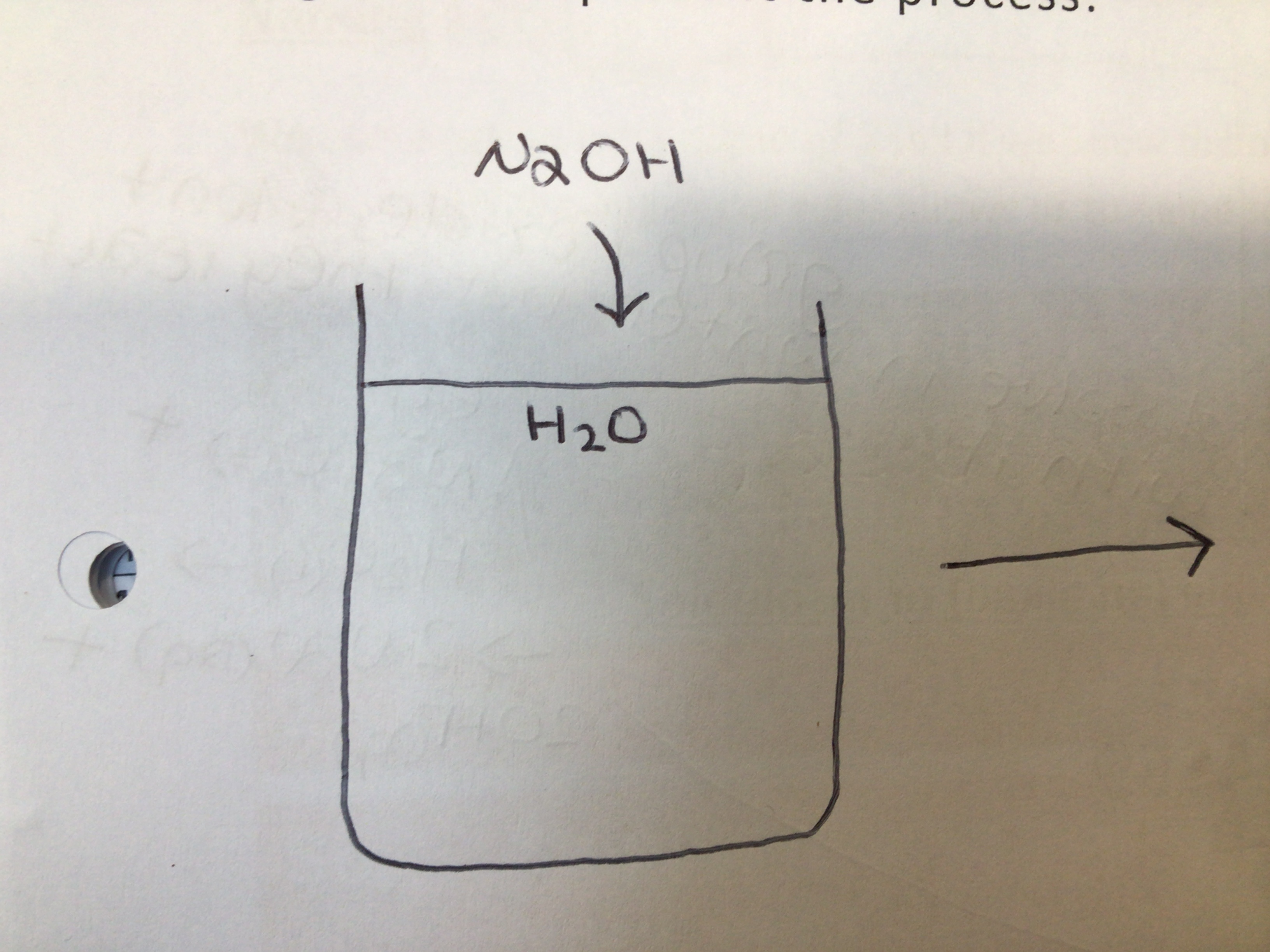
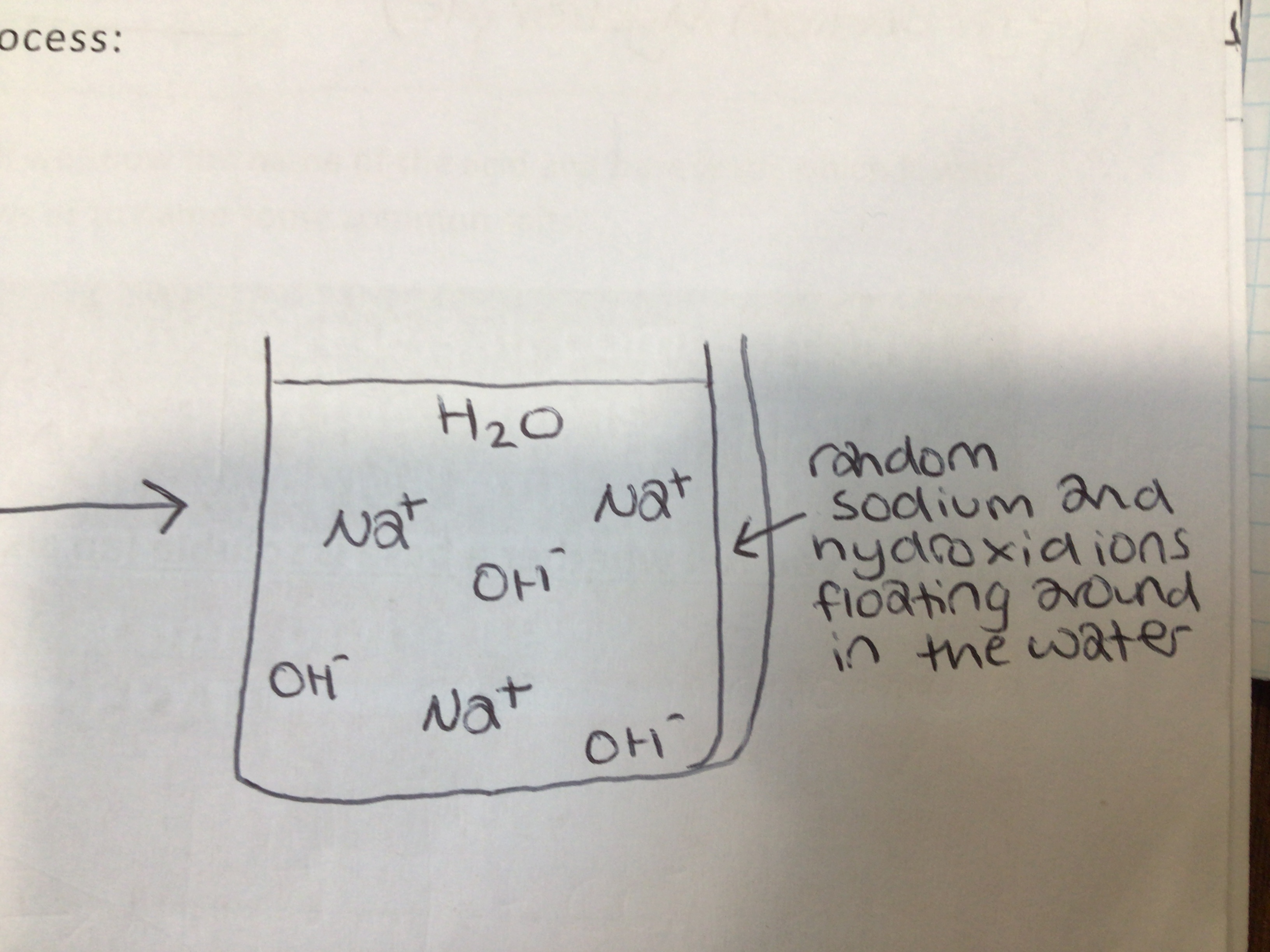
Alkalis form OH- ions when added to …
Water
A solution is alkaline because of the presence of…
OH-
Types of bases:
Metal oxides
Metal carbonates
Metal hydroxides
Ammonia
How can you tell if a base is alkali or insoluble base
Chlorides are made from what acid
Hydrochloric acid
Sulphate is made from what acid
Sulphuric acid
Nitrates are made from what acids
Nitric acid
Ethanoates are made from what acid
Ethanoic acid