calorimetry
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
16 Terms
Specific heat capacity
is the amount of energy required to raise the temperature of 1 gram of a substance by one
kelvin
To convert q into ΔH, we need to check…
the sign that we use. For example, for an
exothermic reaction we use a negative sign, and for an
endothermic reaction we use a positive sign.
calorimetry
heat measurement.
calorimeters
the devices that we use to measure a change in temperature to calculate a change in heat energy
Calorimeter A has a beaker containing 200g of water. Calorimeter B has a beaker containing 250g of water. Calorimeter B would lose less heat energy. How do we know?
The heavier the water, the smaller the increase in temperature. When the water is closer in temperature to its surroundings, it’ll lose less energy. Therefore, Calorimeter B must have a greater mass.
To calculate the enthalpy change of a reaction where a substance is burned or combusted we use a…
flame calorimeter
How can you reduce heat loss in a flame calorimeter?
we could increase the mass of water in the calorimeter or use a bomb calorimeter
To measure the enthalpy change of a reaction in solution, we use a…
constant pressure calorimeter
Which type(s) of calorimetry is used to determine the enthalpy change when liquid propane is burned?
Flame calorimetry
Bomb calorimetry
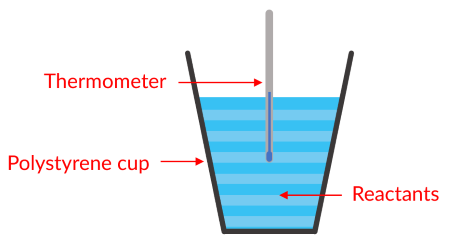
A student wants to calculate the enthalpy change when hydrochloric acid reacts with sodium hydroxide solution. What apparatus should they use?
As both reactants are in solution, constant pressure calorimetry should be used.
The student won’t have access to expensive, high-tech equipment. So, they should mix the reactants inside a polystyrene cup and measure the temperature change with a thermometer.
A simple flame calorimeter loses heat energy to the surroundings. How can the accuracy of the enthalpy change calculation be improved? (2)
Use a larger volume of water
Use a bomb calorimeter instead
Why does increasing the volume of water in a flame calorimeter improve the accuracy of the results?
The larger the mass of water, the smaller the increase in temperature during the reaction.
If water is closer in temperature to the surrounding air, less heat energy escapes.
More of the heat energy released from burning the fuel is transferred to the water.
The heat energy we measure transferred the water is more accurate as less is lost to the surroundings.
What is the density of water?
1gcm-3
To find the molar enthalpy of a reaction…
divide the enthlapy change of the reaction by the amount of moles of reactant
We report the molar enthalpy change in…
kJmol-1