Quiz 4 Chap 5
1/38
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
39 Terms
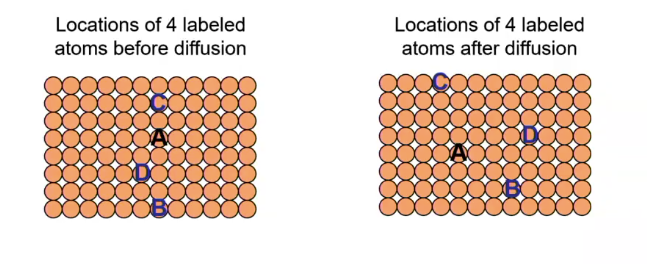
Diffusion
Mass transport by atomic motion relative to neighbors
Gasses & liquids
random (Brownian) diffusion
Solids
vacancy diffusion & interstitial diffusion
Interdiffusion
Migration of atoms from one material to another
Self-diffusion
atomic migration within a pure material
random (Brownian)
thermally driven
doping defined
Diffusion of very small concentrations of impurity atoms (ex. P) into the semiconductor silicon

process of doping
deposit P-rich layers on surface
heat treat the sample to drive in P
Result is P-doped semiconductor silicon
Case hardening of iron alloy
Outer surface selectively hardened by diffusing carbon atoms into surface
Improves wear resistance of gear
Improves resistance to fatigue failure
Vacancy diffusion
atoms & vacancies exchange position
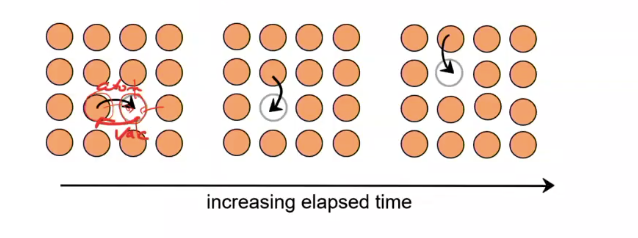
In vacancy diffusion, the diffusion rate depends on (2 things)
activation energy to exchange
number of vacancies
Interdiffusion happens IF
within solubility limit
In interdiffusion, atoms tend to migrate from regions of ____ to regions of _____
high concentration, low concentration
Interstitial diffusion defined
small, interstitial atom move from one interstitial position to an adjacent one
Interstitial diffusion depends on _____ to move to adjacent site
activation energy
Interstitial diffusion is _____ than vacancy diffusion
more rapid
kinetics
how fast underlying processes occur
driving force
provides direction, compels a reaction or process forward
Activation energy, Q
energy required for an atom to break bonds and jump
value depends on material
given element and situation, Q doesn’t depend on T
“jumping” in atoms
when an atom moves from one lattice site to another
Thermal energy available (kT) depends on
Temperature, T
ratio between Q and kT part of
what determines how frequently atoms dump
Diffusion coefficient increases with _____
increasing T
D = D0e-Q/RT
D = diffusion coefficient (m2/s)
D0 = pre-exponential (m2/s)
Qd = activation energy (J/mol or eV/atom)
R = gas constant = 8.314 J/mol*K or k(8.62×10-5 eV/atom*K)
T = absolute temp (K)
Diffusion Coefficient, D
Indication of how fast atoms move in given conditions
related to # of jumps / second
_______ and _______ can contribute to how fast an atom can move, and is reflected in ___
Crystal structure
electronic configuration
pre-exponential, D0
Activation energy, Q and the pre-exponential, D0 do NOT depend on
Temperature, T
If Q/kT ratio is high
lower jump rate
Relationship between diffusion coefficient, D, and Temperature, T
D has an exponential dependence on T
concentration gradient
driving force for diffusion
Assumes T and stress are uniform throughout sample
assumes diffusing species is soluble in host
If concentration (high or low) is uniform throughout a sample
no net transport
random motion in a sample means ___ and results in ______
spread
net transport from high to low concentration region
Diffusion is a ______ process
time-dependent
rate of diffusion (diffusion flux, J): J = M/At
M = mass of diffused species
A = area
t = time
Units: kg/m2s
FIck first law of diffusion
Flux (J) is proportional to concentration gradient
J = -D(dC/dx)
dC/dx = concentration gradient
Steady state diffusion
Concentration of an atom does not change at a given location over time
In Steady state diffusion, flux must be ________
constant over entire length of piece
In steady state diffusion, flux (J) is independent of
time
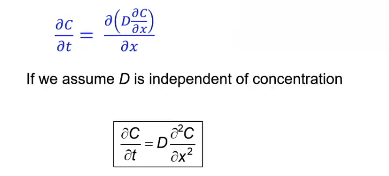
Non-steady state diffusion
Concentration of diffusing species is a function of both time and position C = C(x, t)
seek solutions to Fick’s 2nd law — assume D is independent of concentration
kinetics — how fast atoms jump
Increasing T → Increasing TE & T → activation energy is reached more frequently → increasing vacancy concentration