CHEM 221: Midterm Concepts and Vocabulary (pt. 1)
1/86
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
87 Terms
quantitative analysis
how much of a chemical substance is present
qualitative analysis
what chemical substance is present
ordinate
y-axis
abscissa
x-axis
titrimetry
method based on a measured volume
gravimetry
methods based on measured weight
electrochemical analysis
rely on measurement of potential, current, resistance and charge
spectral methods
interaction of an analyte with electromagnetic radiation (spectrophotometry, spectroscopy)
chromatography
separation of materials due to its interaction with two different phases
chemometrics
statistical treatment of data, how we deal with and process data
steps for a typical quantitative analysis
choosing a method
acquiring a sample
processing the sample
eliminating inferences
calibrating and measuring concentration
calculating results
evaluating and estimating their reliability
complete analysis
the goal is to determine the amount of each component in a sample
partial analysis
the goal is to determine the amount of one or a limited number of components (without regard for total composition)
factors to consider before an experiment
accuracy and sensitivity, cost, number of samples to be assayed, number of components in a sample
mass vs. weight
mass never changes, weight changes based on gravitation force
buoyancy correction
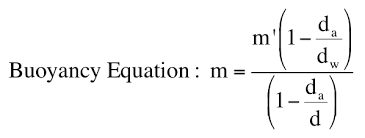
how to read pipettes, burets, and graduated cylinders
make sure the apparatus is at eye level, the lower point is the volume
solute
minor species in solution
solvent
major species in solution (dissolves the solute)
percent concentration (wt%)
grams of solute per 100 grams of solution
molarity
moles of solute per liters of solution
molality
moles of solute per kilograms of solvent
analytical molarity
total # of moles of a solute in 1L
equilibrium molarity
molar concentrations of particular species after dissociation
strong electrolyte
substances completely dissociations into ions in a solution
weak electrolyte
substance only partially dissociates into ions in solution
volume percent (vol%)
(volume of solute/total volume of solution) x 100
ppm
(mass of substance/mass of sample) x 10^6
specific gravity
ratio of a substance mass and the mass of an equal volume of water
accuracy
the closeness of the measurement to the target or reference value
precision
the repeatability or consistency of a measurement
error
measurement for accuracy, E = measured - target
relative error
(measured-target)/target x 100
relationship between repetitions and error
the more measurements you make, the closer you will get to the average which means the error will be decreased
addition and subtraction sig figs
only digits after the decimal point matter
multiplication and division sig figs
all digits matter
systematic/determinate errors
problem with the methods, all errors are of the same size, magnitude and direction
random/indeterminate errors
based on limits and precision of a measurement, can be treated statistically
examples of determinate errors
potential instrumental errors (variation in temp, contamination of the equipment, component failure), method errors (slow or incomplete reaction, unstable species, side reactions), personal errors
when is indeterminate error present?
ALWAYS
relative error %
(loss or excess/true quantity) x 100
number of measurements and confidence limits relationship
increase the number of measurements, decrease the confidence limits
confidence interval
the range of values within which the population mean is expected to lie with certain probability
confidence level
the probability that the true mean lies within a certain interval, usually expressed as a %
what are linear regressions often used for?
calibration purposes
t-test case 1
compare a small sample mean with a true mean
t-test case 2
compare two sets of measurements
t-test case 3
compare individual differences
three common approaches for quantification
calibration curve, standard addition, internal standard
why do you want a higher slope for a calibration curve?
higher slope value means it has a higher sensitivity
calibration sensitivity
m, the slope of the linear regression
limit of detection
(3.33 x standard deviation of the blank)/slope of the calibration curve
limit of quantitation
(10 x standard deviation of the blank)/slope of the calibration curve
why is standard addition a good calibration technique?
it minimizes the impact of matrix effects by adding known concentrations of the analyte directly to the sample ensuring the standard and unknown samples have identical matrices
internal standard process
add a known amount of a standard analyte similar to the analyte of interest to the sample, measure response of analyte and standard
response factor
compares the signal produced by a substance to the amount of substance that produced the signal
nonelectrolyte
compounds that are soluble in water but do not dissociate in ions (no increase in conductivity is observed)
arrhenius acid
produce hydronium ion in aqueous solutions
arrhenius base
produces OH- in aqueous solution
bronsted-lowry acid
proton donor
bronsted-lowry base
proton acceptor
lewis acid
electron acceptor
lewis base
electron donor
autoprotolysis
chemical reaction where a proton is transferred between two identical molecules
strong acid and bases
completely dissociate in aqueous solutions
weak acids and bases
incompletely dissociate in aqueous solutions
lechatlier’s principle
have a system that is in equilibrium
subject the system to change
equilibrium is disturbed
system proceeds back to equilibrium such that the change is offset
K (equilibrium constant)
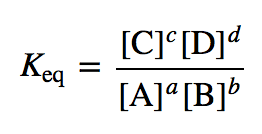
Kw (ionic product of water)
= [H3O+][OH-]
Ksp (solubility product)
used for ionic materials that are slightly soluble in water (no solids)
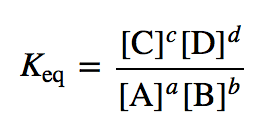
constant of formation (Kf)
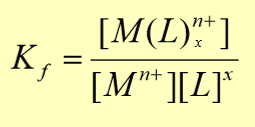
constant of decomposition
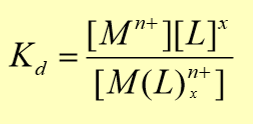
complex formation
two or more species join together to form a single species
equilibrium constant of acids (Ka)
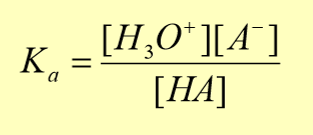
equilibrium constant of bases (Kb)
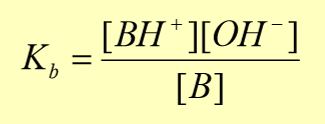
dissociation for conjugate acid/base pairs
Ka x Kb = Kw
effect of ion charge on equilibria
electrostatic forces cause each ion from a dissociated species/reactant to be surrounded by a “sheath” of oppositely charged ions from the added electrolyte, this reduces ion affinity, increases solubility
electrolyte effect
affinity of the ion is reduced
solubility increases as more ions are “pulled” into the solution
the greater number of electrolyte ions, the greater the effect
in dilute solutions, “sheaths” are not likely
ionic strength
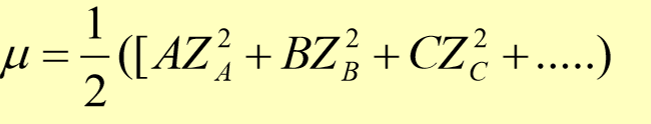
activity coefficient
“effective” concentration of a species depends on the ionic strength
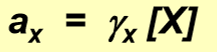
properties of the activity concentration
measures the “effectiveness” of a species in an equilibrium, independent of the nature of the electrolyte
assumed 1 for unchanged molecules
the more charge an ion carries, the faster it will change from 1
dubye-huckel equation
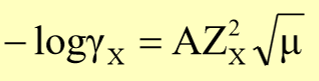
extended debye-huckel equation
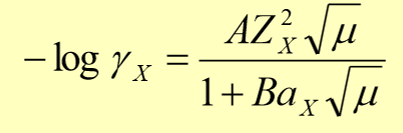
debye-huckel equation for the mean activity coefficient
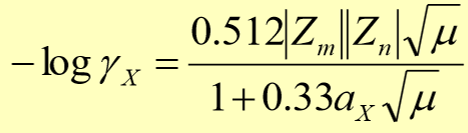
limits for debye-huckel equation
only works for extremely dilute solutions, it ignores ion-dipole interactions
analyte
substance whose concentration is being determined (unknown)
titrant
solution of known concentration that is added to an analyte to determine its concentration