3.1.3.7 forces between molecules
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
12 Terms
What is the difference between covalent bonds and intermolecular forces
C.b are bonds between a toms
I.f are attraction between molecules
Dipole dipole forces act between molecules that have
Permanent dipole
Dipole dipole is stronger than
Vandwerwaal
What is the most common i.f
Van dear walls
How do temopoary dipoles form
At any moment the electrons can be anywhere and so the distribution of charge is changing at any instant
A temporary dipole is formed it only happens for a moment but there always is one
As electron distribution changes it will induce new dipoles in atoms around it
How do van der waals occur
Caused by changing position of electron
So the more electrons thee larger the inatntsous dipole is
What are the 3 eemtnts that must be present for hydrogen bond to occur
N nitrogen
O oxygen
F fluorine
One of them also must have a lone pair of electrons
Hydrogen bond is stronger than bu weaker than
Stronger then dipole dipole
But still weaker than covalent bond
Describe the hydrogen bonding in water
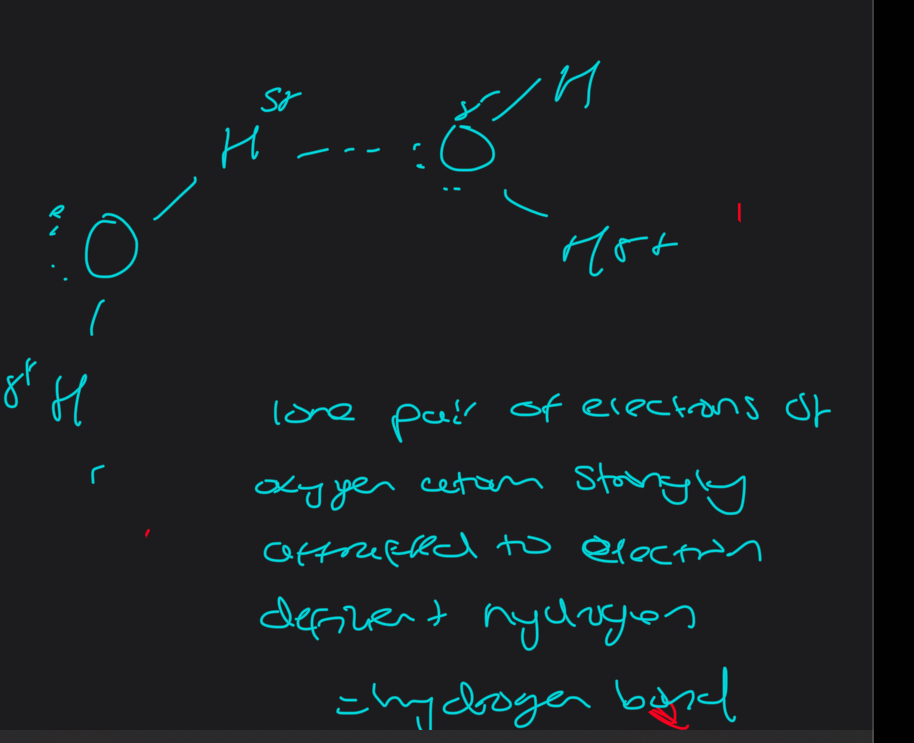
What do you have to make sure the hydrogen bond is
Linear
What is the importance of hydrogen bonding in the low density of ice
In water the hydrogen bonds break and reform easily when water freezes the wate molecules no longer free to move about
Hydrogen bonds hold molecules in fixed positions to give it 3d shape
In order to fit into this the molecules are less close together so LESS DENSE