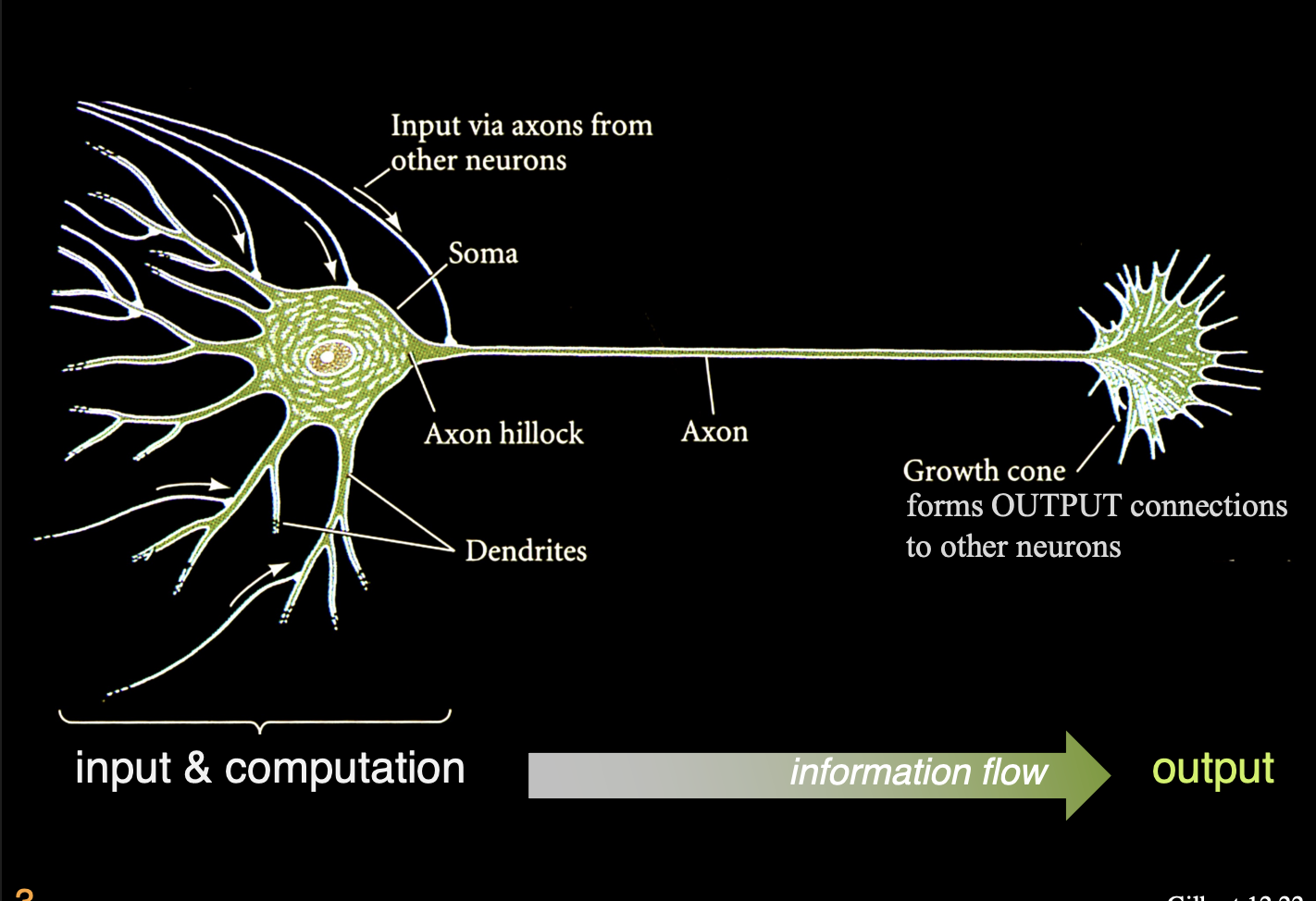
L1: The growth cone
1/50
Earn XP
Description and Tags
The organelle that allows neurons to send processes from one part of the nervous system to another
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
51 Terms
Textbook chapters for this
Development of the Nervous system
Chpt 5, 6, and 8
Learning objectives
What growth cones do
cytoskeletal dynamics underlie navigation
Guidance cures-how these regulate the cytoskeleton
Questions being explored in this lecture
Once different neuronal cell types are specified, each neuron sends out a primary neutrite (the axon) with which to reach the brain regions and connect other neurons
But
How do neurons know which direction
and along which path to extend?
What was the clash of view of Golgi and Cajal
Golgi→ Reticularists
Invented a way of visualising NS with golgi black stain
labeled only a few cells so could see them more clearly
though the nervous system is a reitculum→ a physical continuum extends between nerve cells forming a ‘nrve net’
Cajal→ Neuronists→ each neuron is an independent cell

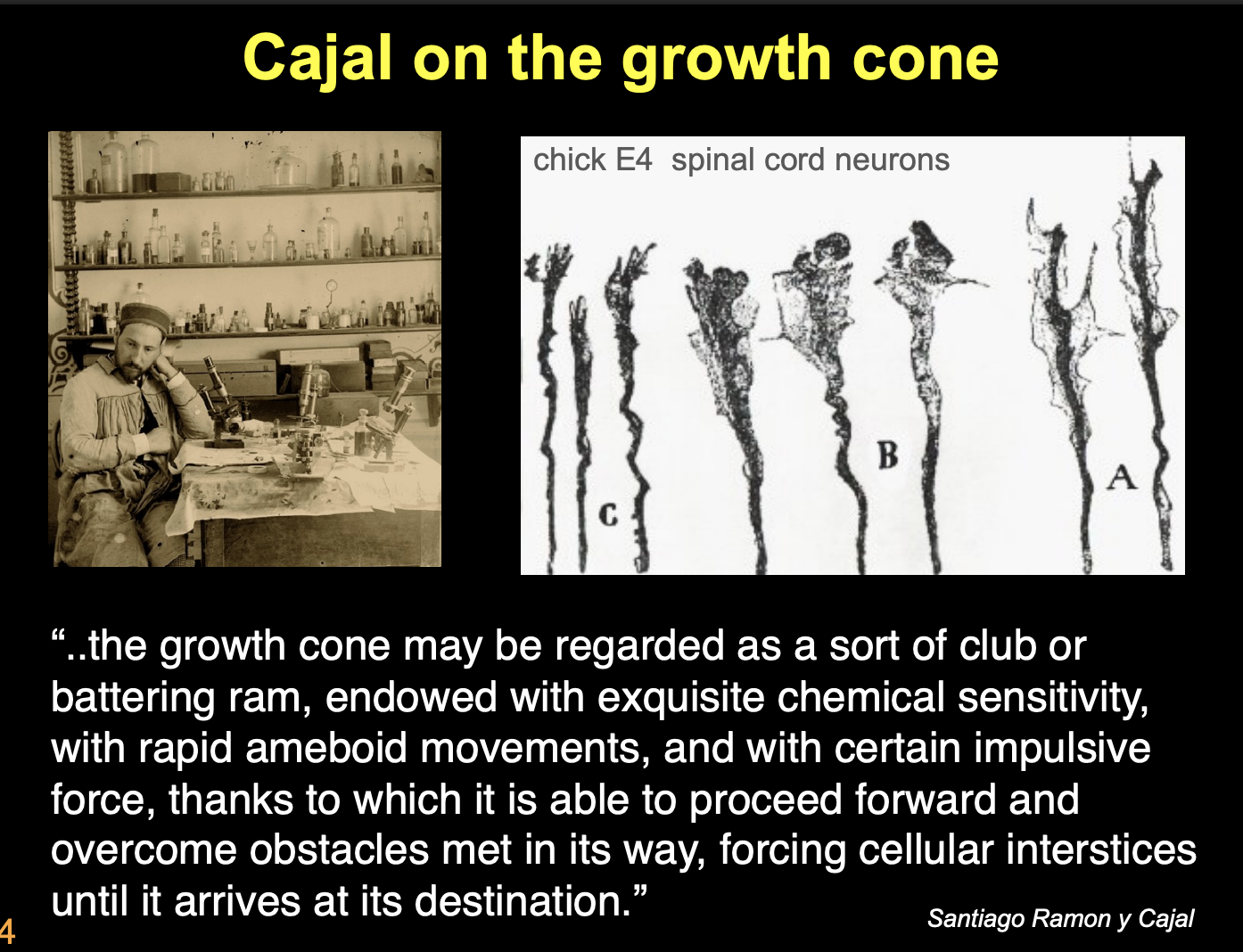
What did Cajal’s work on fixed embryonic neural tissue show
described the specialised structures at distal tips of axons→ growth cones
He imaged these as
battering rams with which axons might force their way through the embryonic tissue
cone was a like a club or fingerlike protrusion
force its way through tissue to make connections with other nerve cells
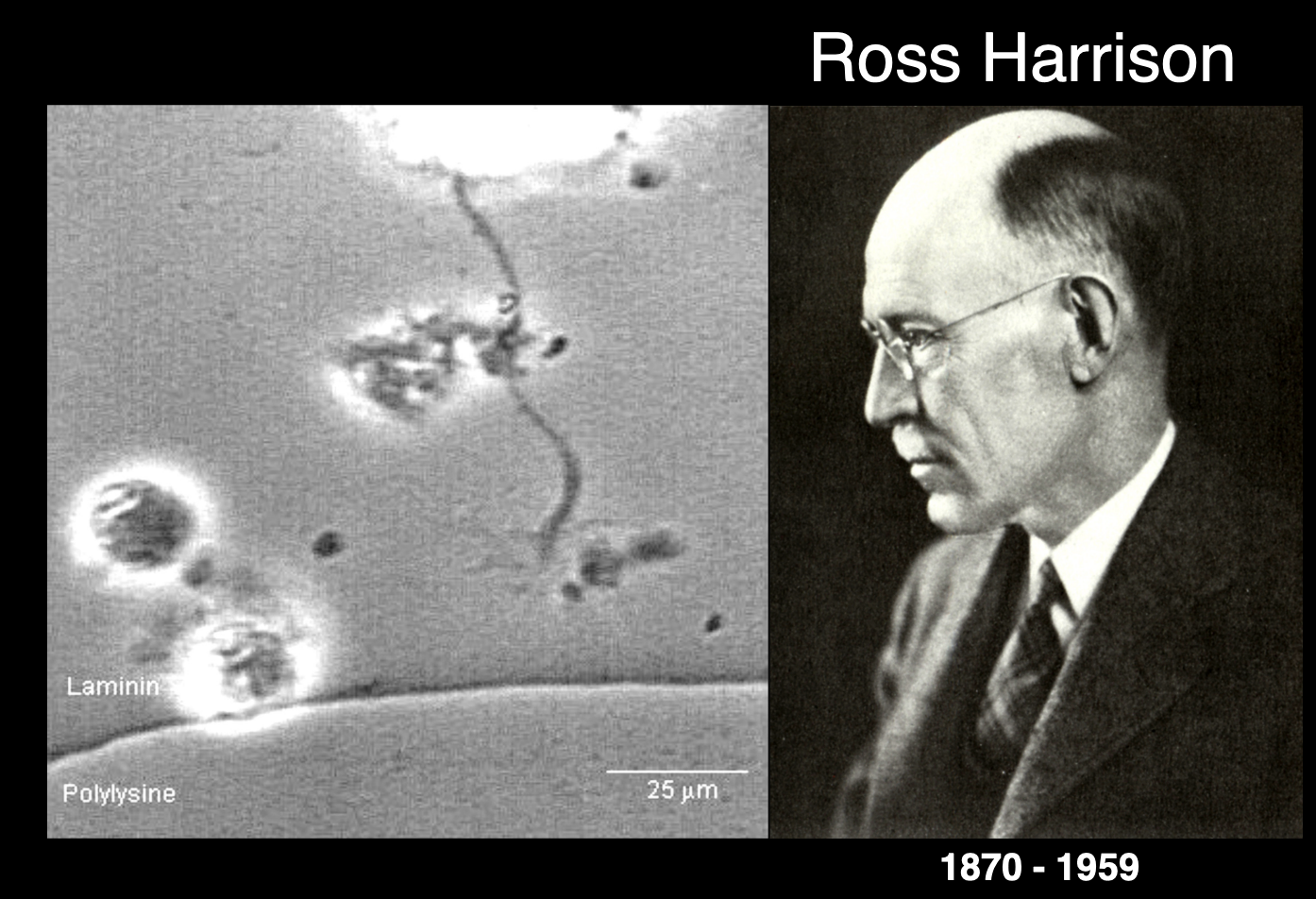
Ross Harrison’s work and observations
Experiment:
Tissue culture→ drop of lymph fluid and hunf upside down
observe live growth cones in real time using cultured pieces of embryonic neural tube
Observations:
cones are dynamic structures
showed motile contractile forces and pulling→ on laminin bead
hand-like appearance→ equipped with finger-like filopodal extnesions
these are continuously sent out and rapidly retracted
(as if being used to sample the environment)
Membranous lamellipodia extend between filopodia
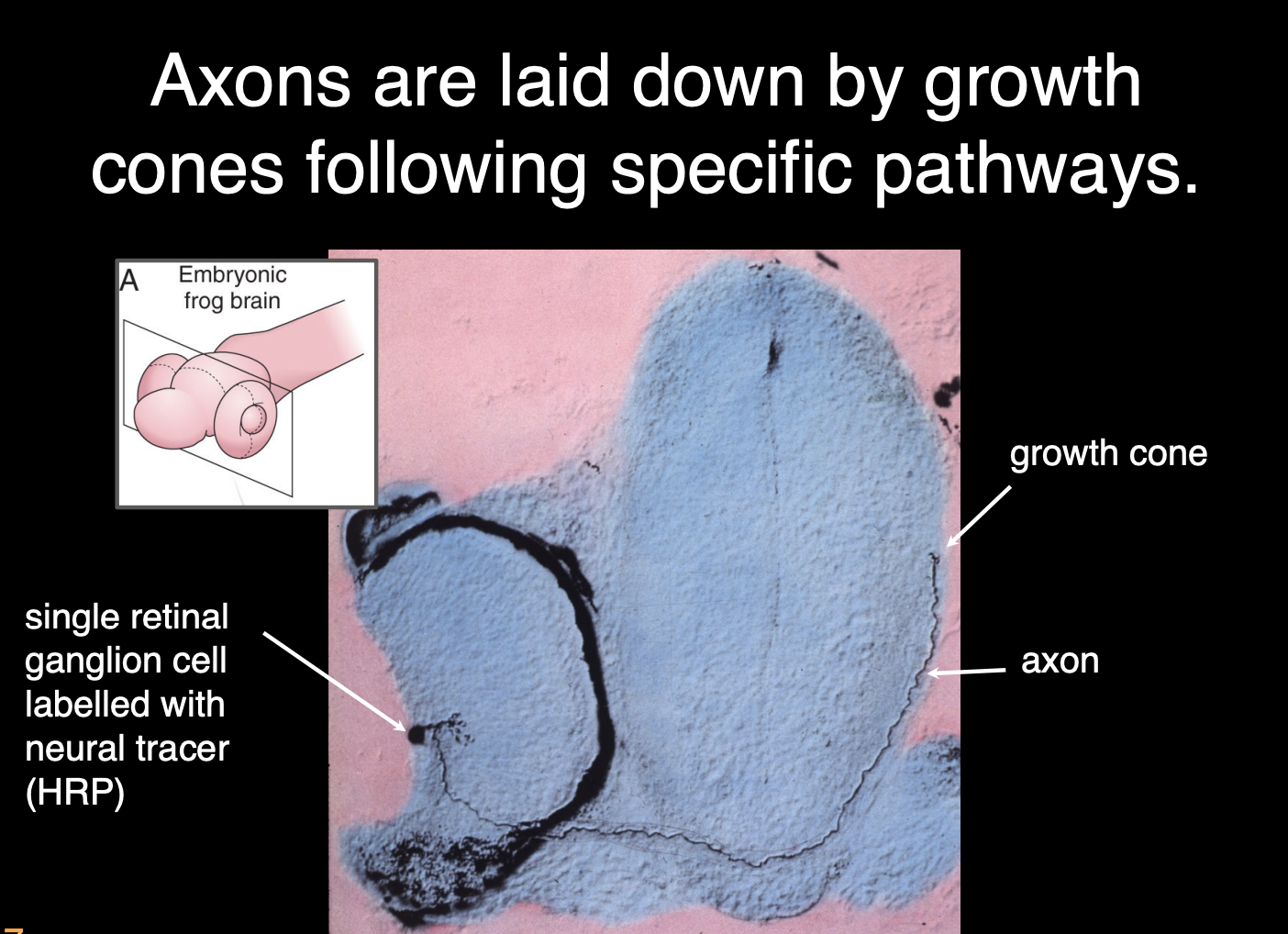
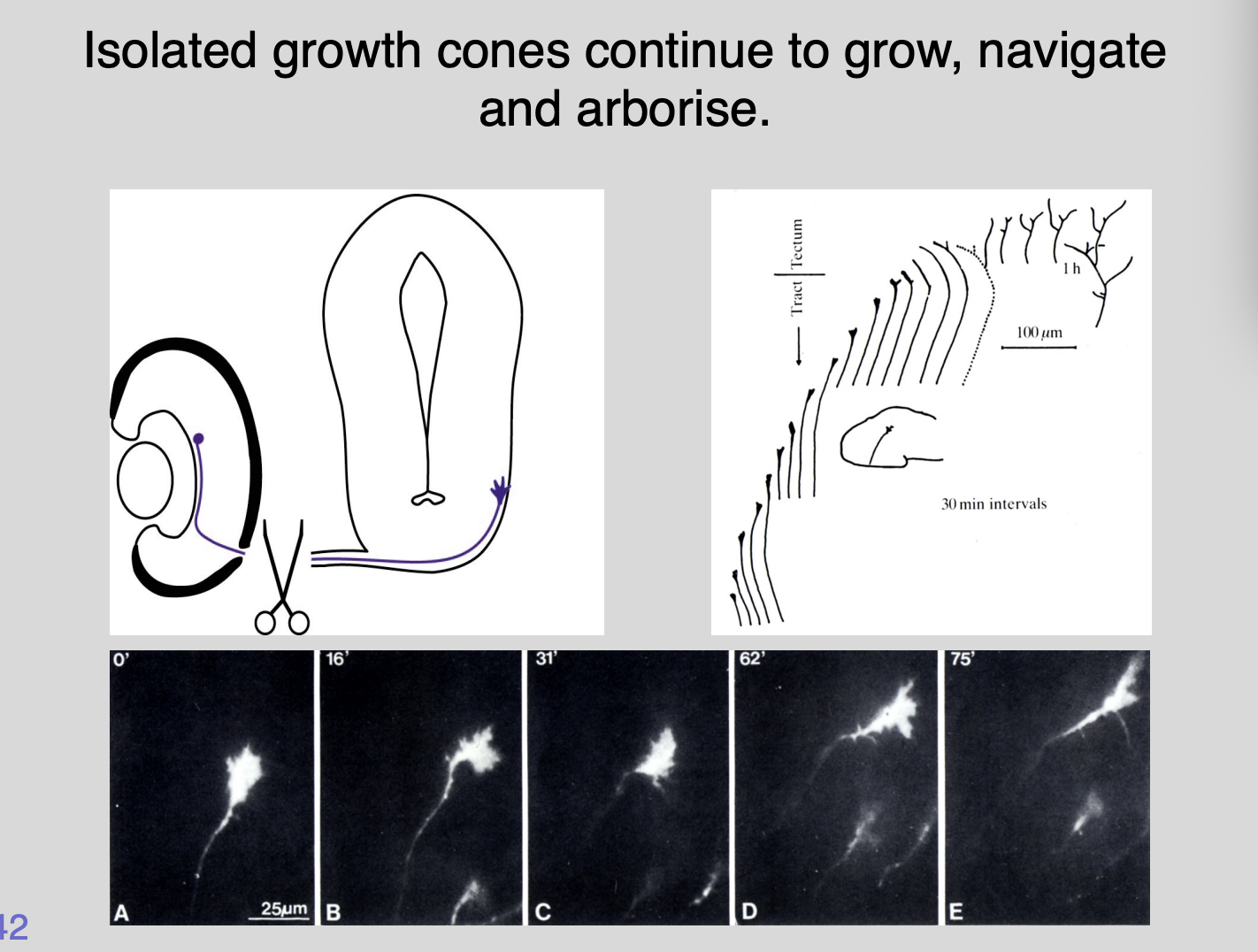
Work and observations of William Harris of growth cones
Experiment
retinal ganglion cells sent out axons (labeled with neural tracer HRP)
always tipped with a growth cone→ from the retina to the optic tectum
But then the axon was separated from the cell body
Obersvation:
Time lapsed→ growth cone still navigate for several hours
along correct pathway into optic tectum
THEREFORE: shows growth cone has everything it needs for navigation
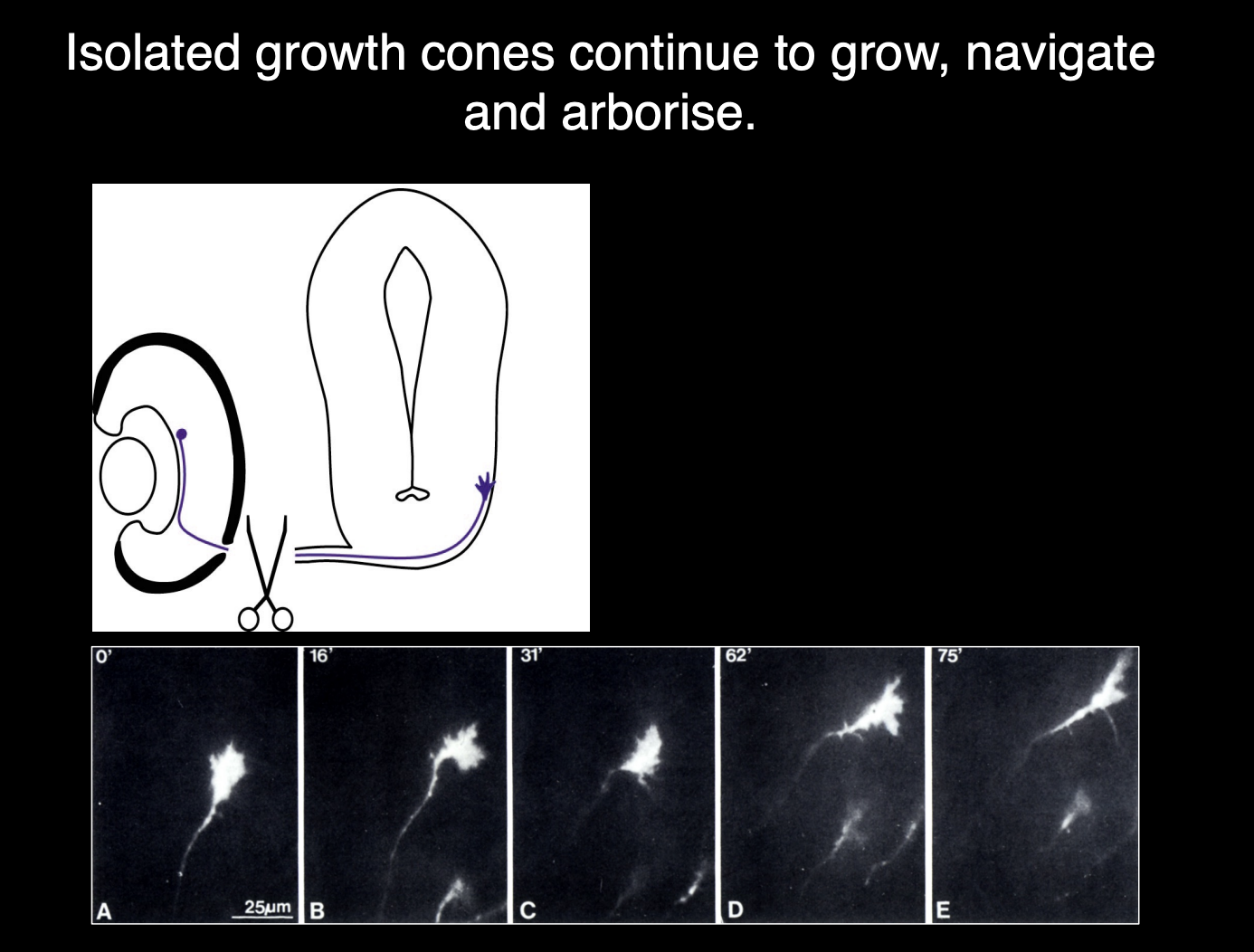
Therefore in understanding the growth cones we can understand
how nerve cells can send their axons from one part to another
→ next things to investigate→
How does it turn left and right? What mechansism?
does it push or pull?
how does it know when to turn left and right?
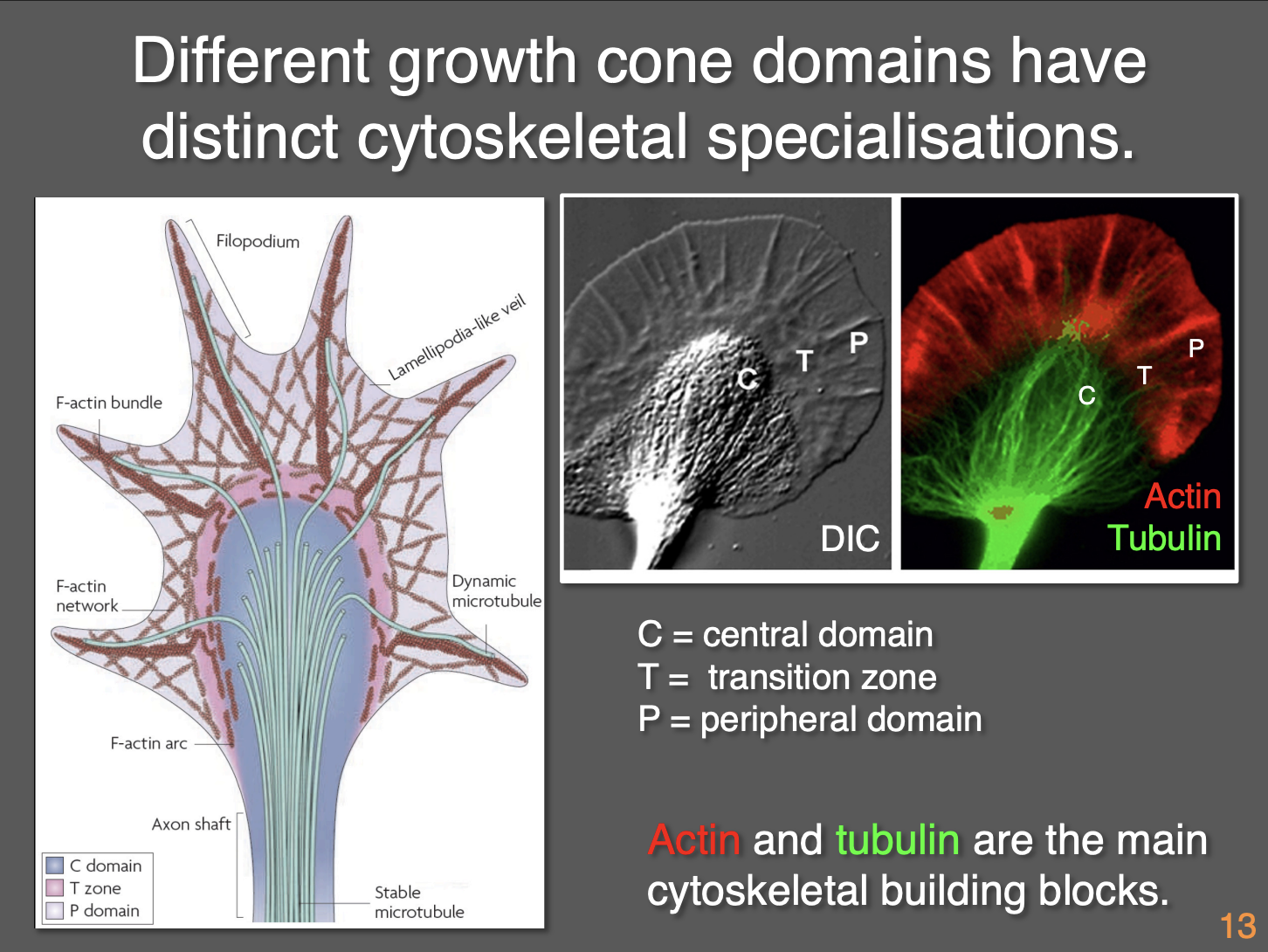
Compartments of the growth cone: 3 Morpholigically distinct domains
Central domain (contains organelles)→where axons terminates
Transition zone (abuts the central)→ shows characteristic membrane ruffling activities
Peripheral domain (distal extent of the transition zone)→ consist of filo and lamellipodia
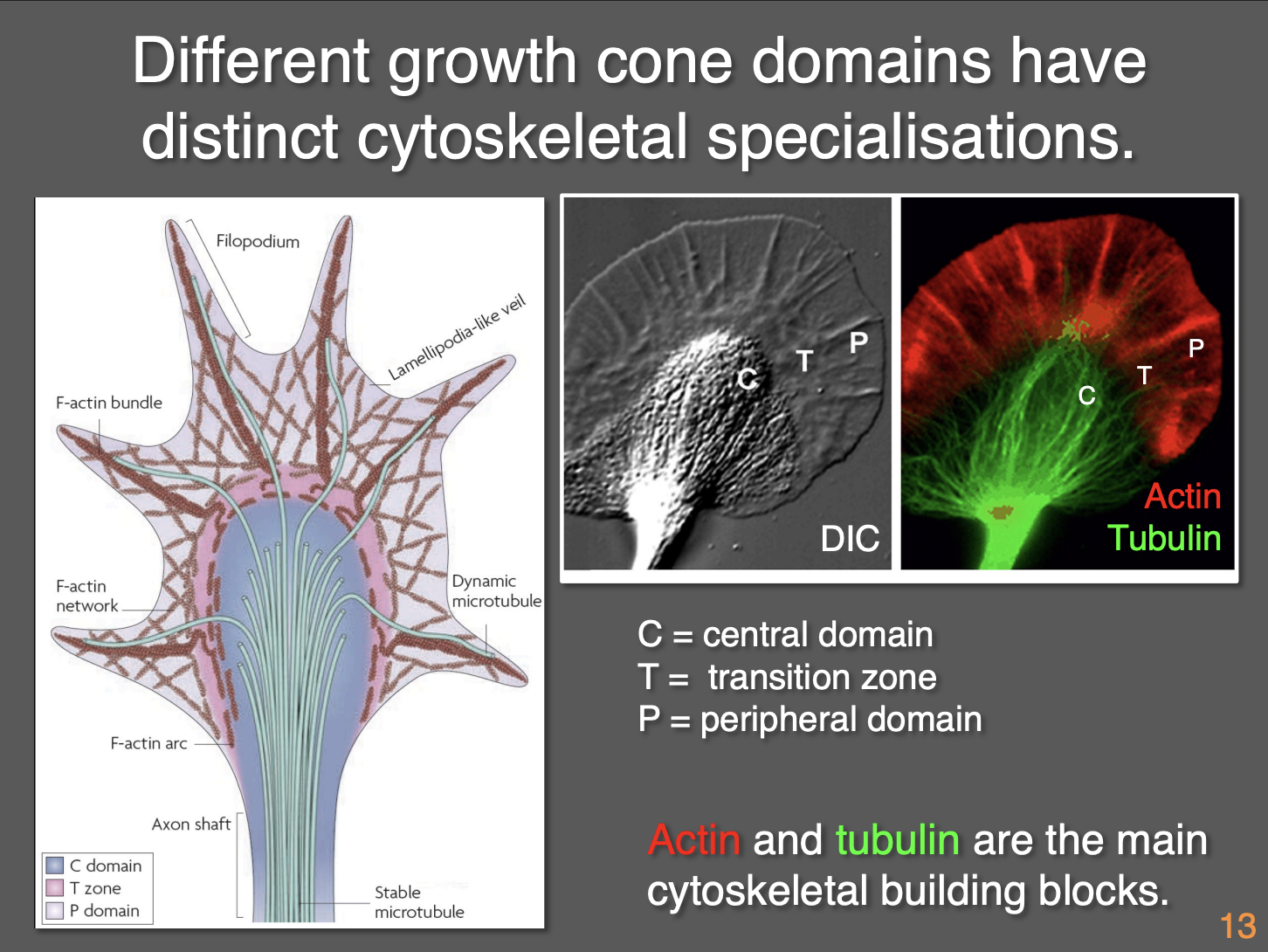
How are these three domains separated
Different distributions of cytoskeleton (actin and tubulin mainly)
Central domain→ bundles of essentially parallel microtubules from the axon invade the central domain → where they fan out
Transition zone→ distal tips (‘plus’ ends) of MT above reach into transition zone
Peripheral domain→ individual MT extend their plus ends into the peripheral domain→ BUT→ primarily contains filamentous actin and few MTs
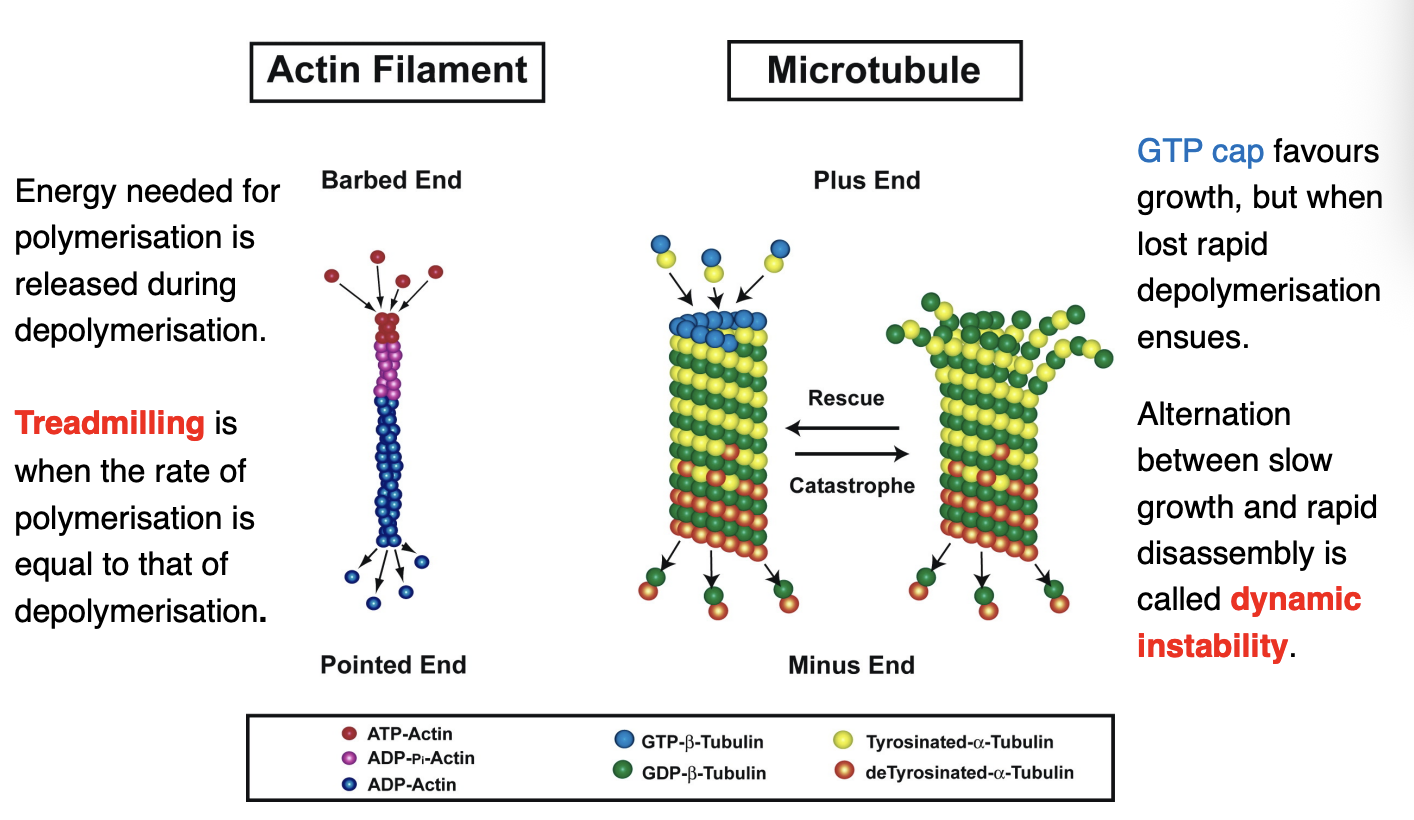
How do growth cones show polarity
Actin and microtubules are key elements are are both polarised
How do the actin and microtubules work for growth
Actin filament
energy needed for polymerisation is released during depolymerisation
Treadmilling→ when the rate of polymerisation is equal to that od depolymerisation
Microtubule
GTP cap favours growth but when lost→ rapid depolymerisation ensures
Alternation between slow growth and rapid disassembly is called dynamic instability
Due to the assymetric components of actin and MT that make the pushing and pulling
What are the general mechanics of growth cone propulsion
Combination of pushing a pulling
Three sets of experiments to show the pushing and pulling
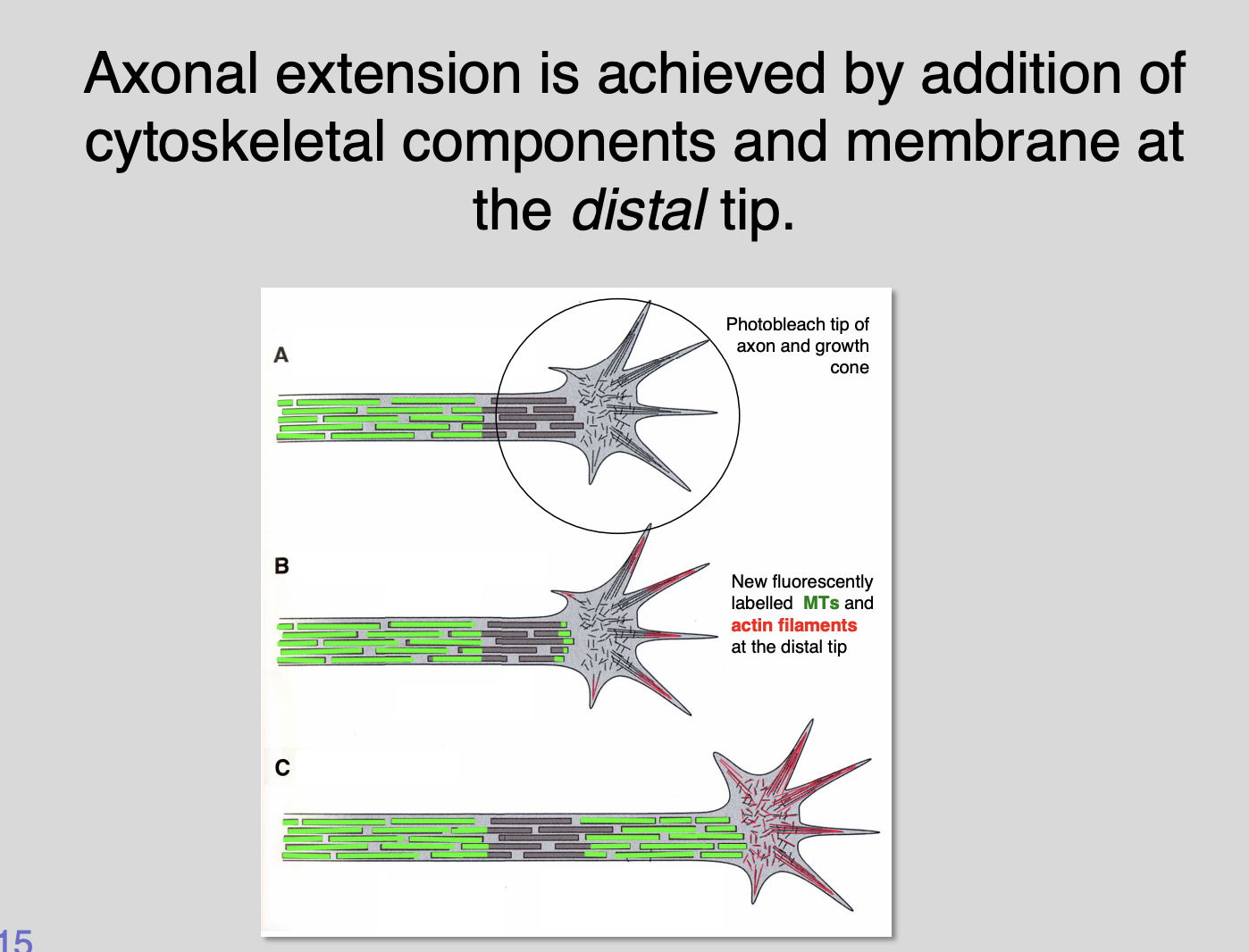
Cultured microtubules fluorescently labelled
if a small spot of fluorescence is photo bleached with laser near distal end→ the bleached spot remains stationary but the axonal extension continues
can see where new radiolabelled components are added
Suggests→ axons extend by inserting new microtubule building components at their distal tips→ PUSHING growth cones forward
Growth cone filopodia cultured neurons
shows tugging on other axons
if individual filopodia lifted off substrate with fine glass needle
Shows→ growth cone snaps into new direction
Suggests→ filopodia exert a tensile force
Actin depolymerising agent cytochalasin-B applied at concentrations where selectively disrupts the formation of filopodia at the growth cone
affects most senstiive part to the drug)
Shows→ slowing down and stabilising of axonal growth
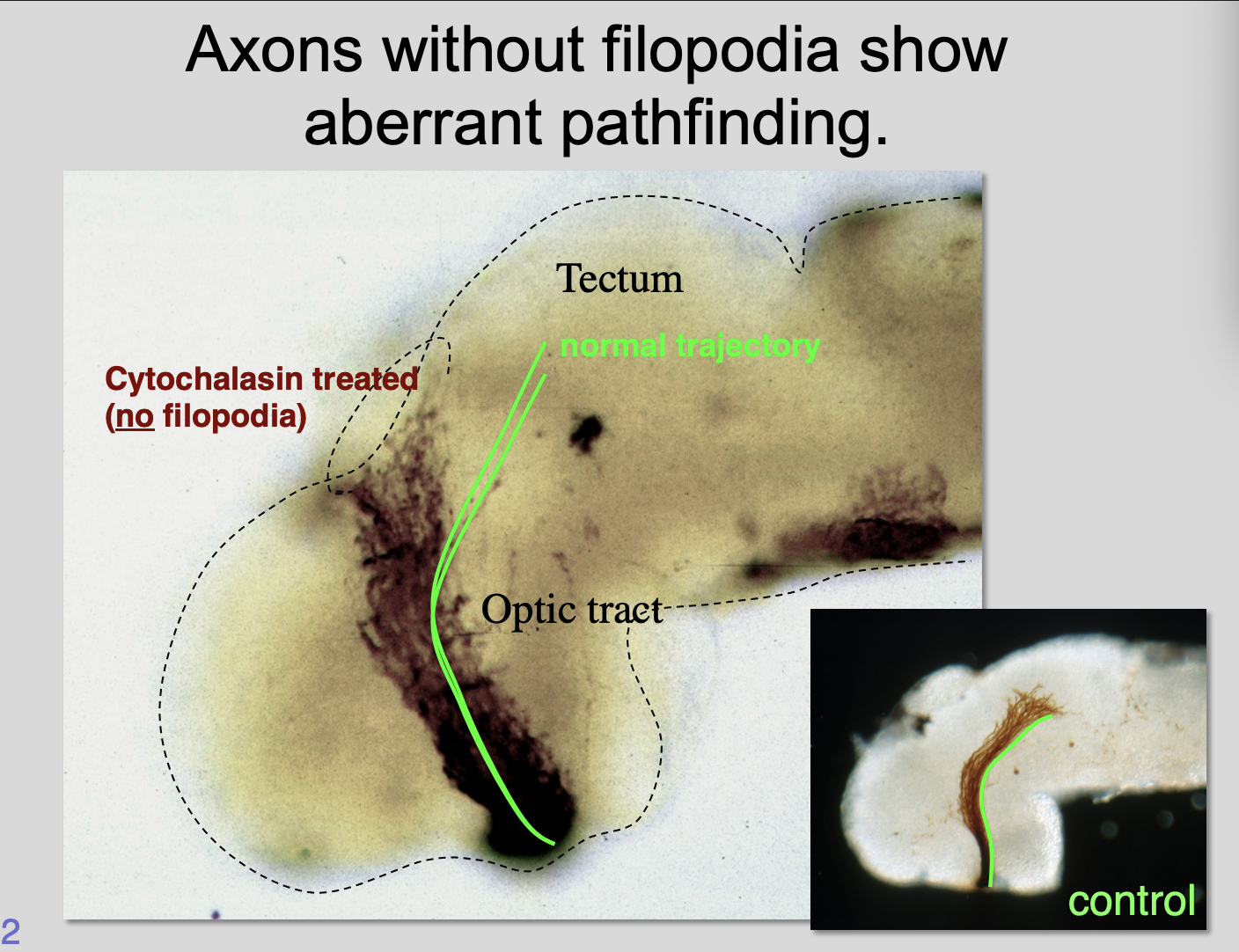
Pathfinding ability is also lost
Overall what do these experiments show as to how filopodia work
probe their environment for directional cues
Pull the growth cone forward
Microtubule polymerisation in the central domain→ may help push it forward
Overall: the extension and retration cycles of filopodia are achieeved by a combination of three independent processes
Rapid actin assembly from G-actin monomers at the leading edge at the tips of filopodia
Myosin-powered retrograde flow of filamentous actin networks from leading edge to the transitional zone
Proximal recyling of filamentous actin inthe transitional zone
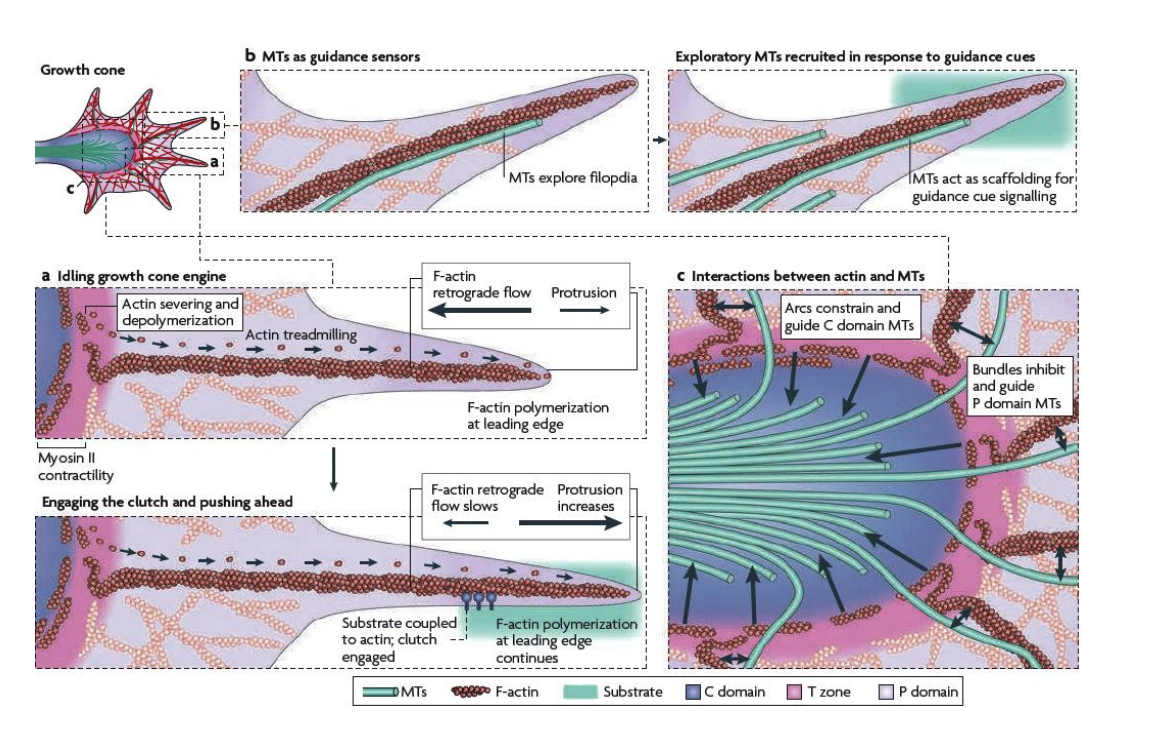
The rate of growth cone advance is determined by
Balance of actin assembly at the leading edge (pushing)
Rate of retrograde translocation of actin filaments towards the transition zone (pulling)
If anything is stopping it→ substrate
Therefore: growth cone advance cold be achieved by
an increase in rate of actin assembly at the leading edge
or
descreasing the rate by which myosin motors drive F-actin retrograde translocation (flow)
Dynamic microtubules are important in growth cone guidance→ what do they do
Constantly extend into the peripheral domain (guided by F-actin)→ into Filopodia
experimental evidence shows:
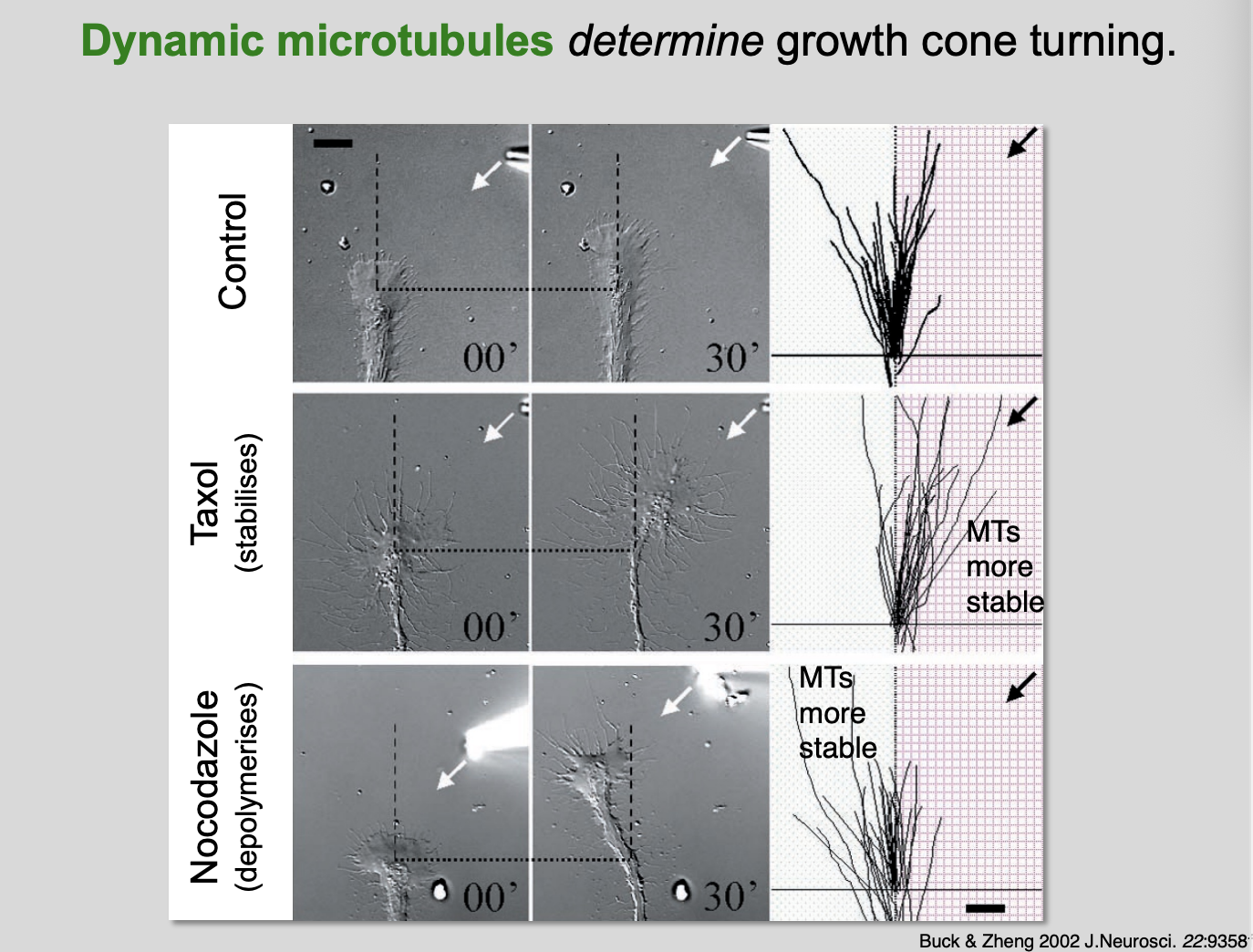
Local stabilisation of dynamic microtubules leads to→ growth cone turning toward the side of stabilisation
Local destabilisaition→ opposite effect
Demonstrates→ local sttabilisaition of dynamic MT is critical for growth cone navigation
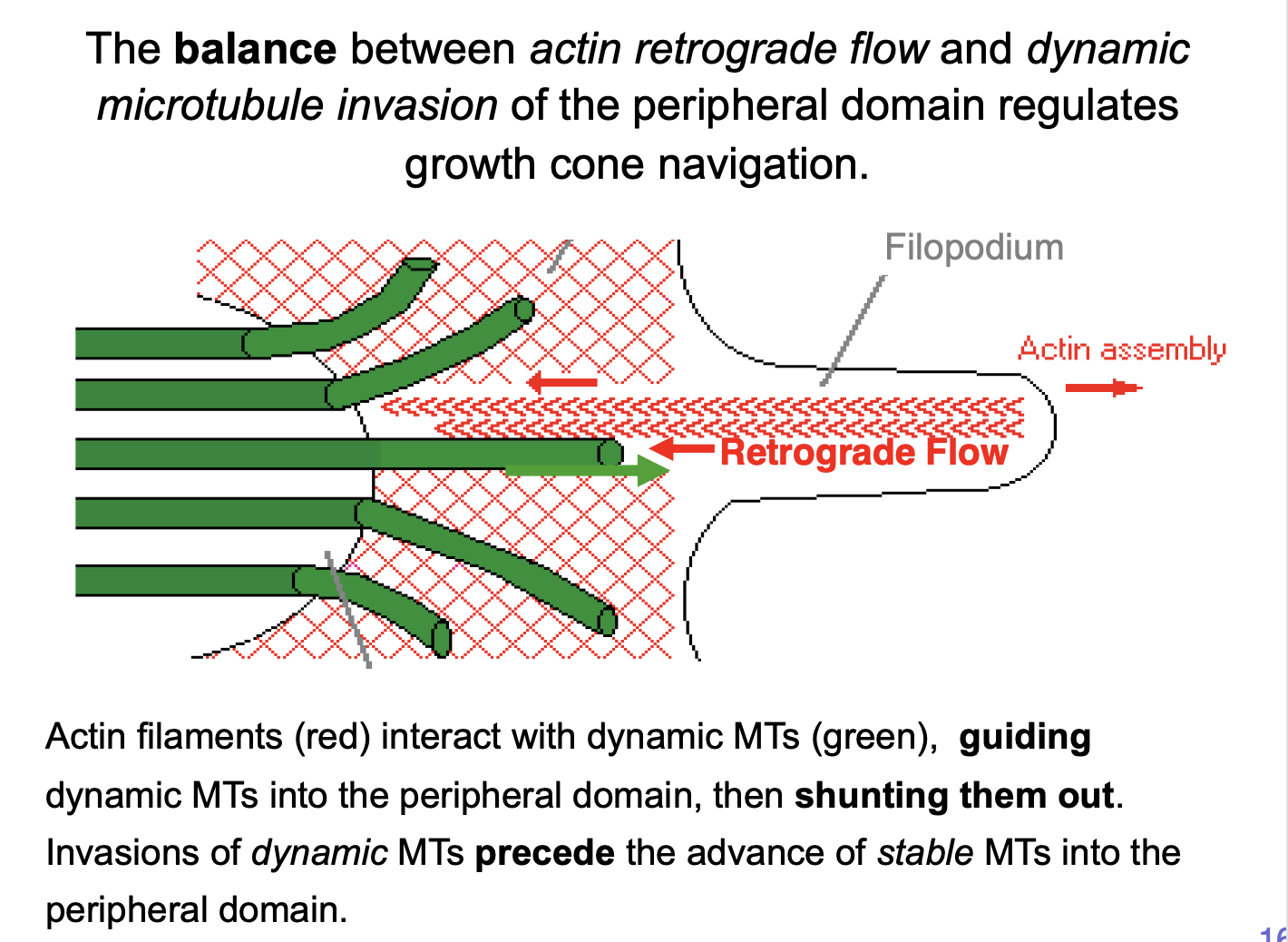
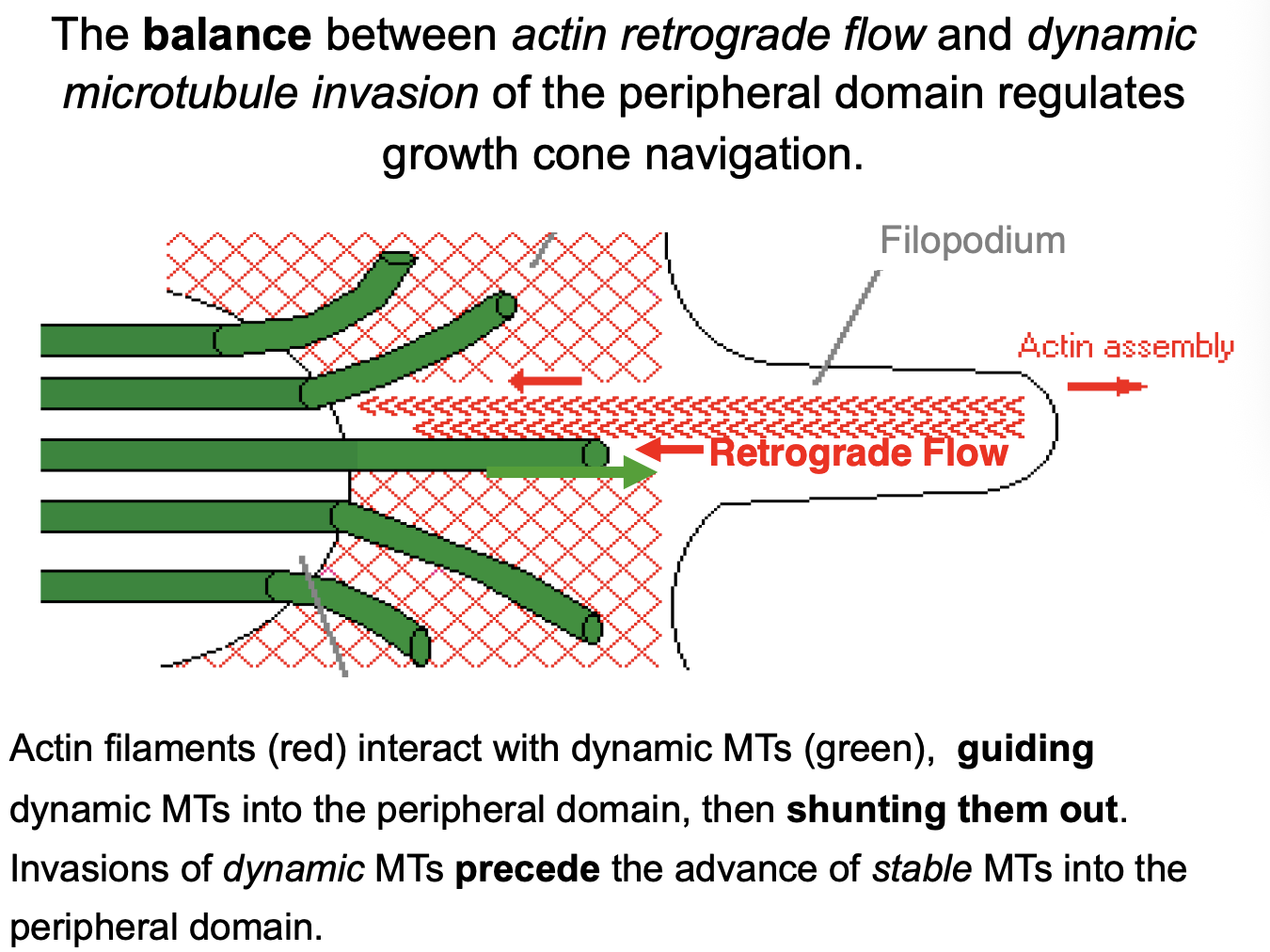
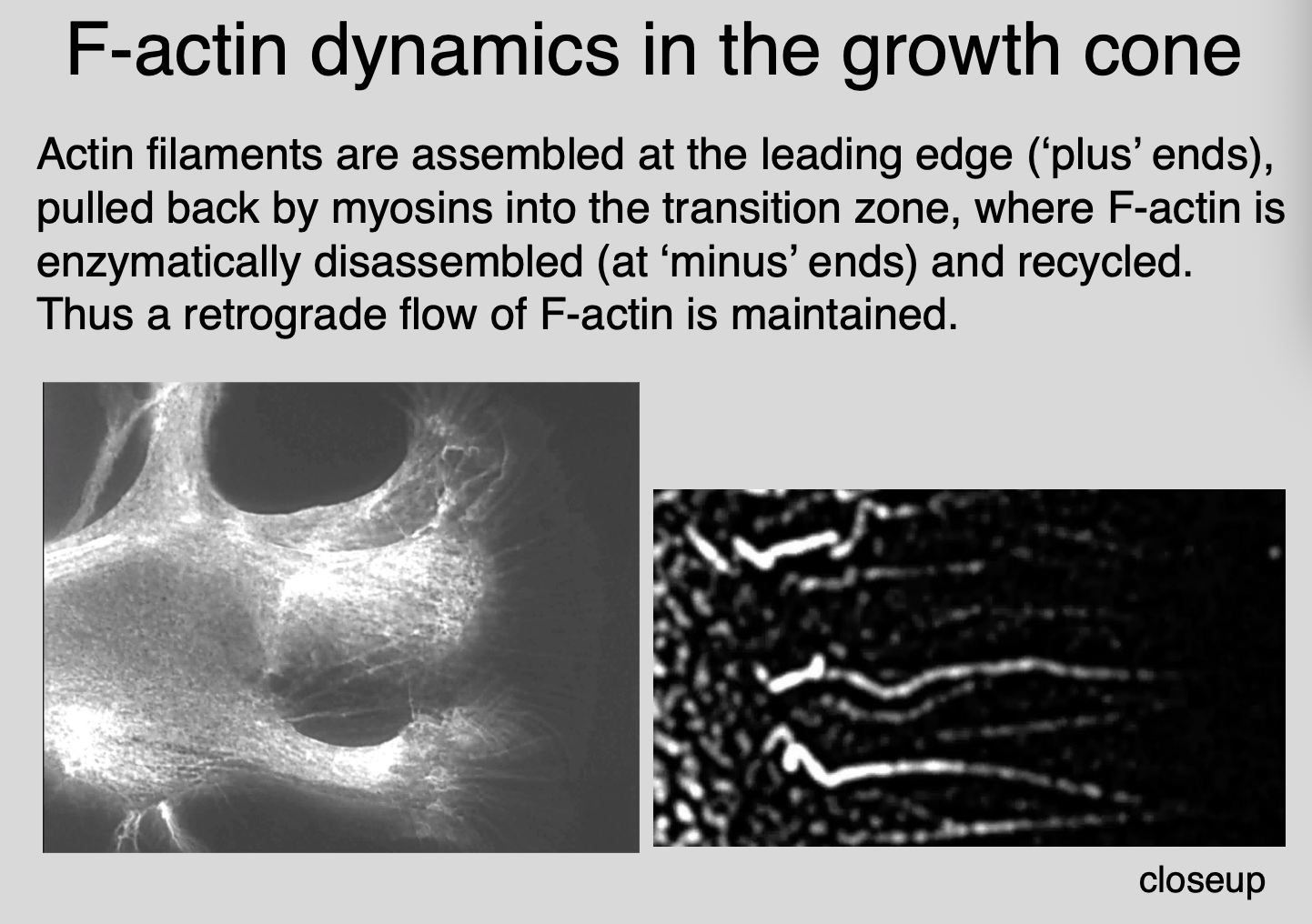
How do these processes cause the growth cones to advance→ actin filaments in peripheral domain flow retrogradely
How do they flow retrogradely:
Assembly at the leading edge (‘plus’ ends)
translocation proximally by myosin motors
(actin is pulled back by myosins into transition zone)
enzyme mediated disassembly/recycling in the transition zone
F-actin is enzymatically disassembled (at ‘minus’ ends) and recyled
→ therefore→ a retrograde flow of F-actin maintained
Image→ can see the retrograde flow
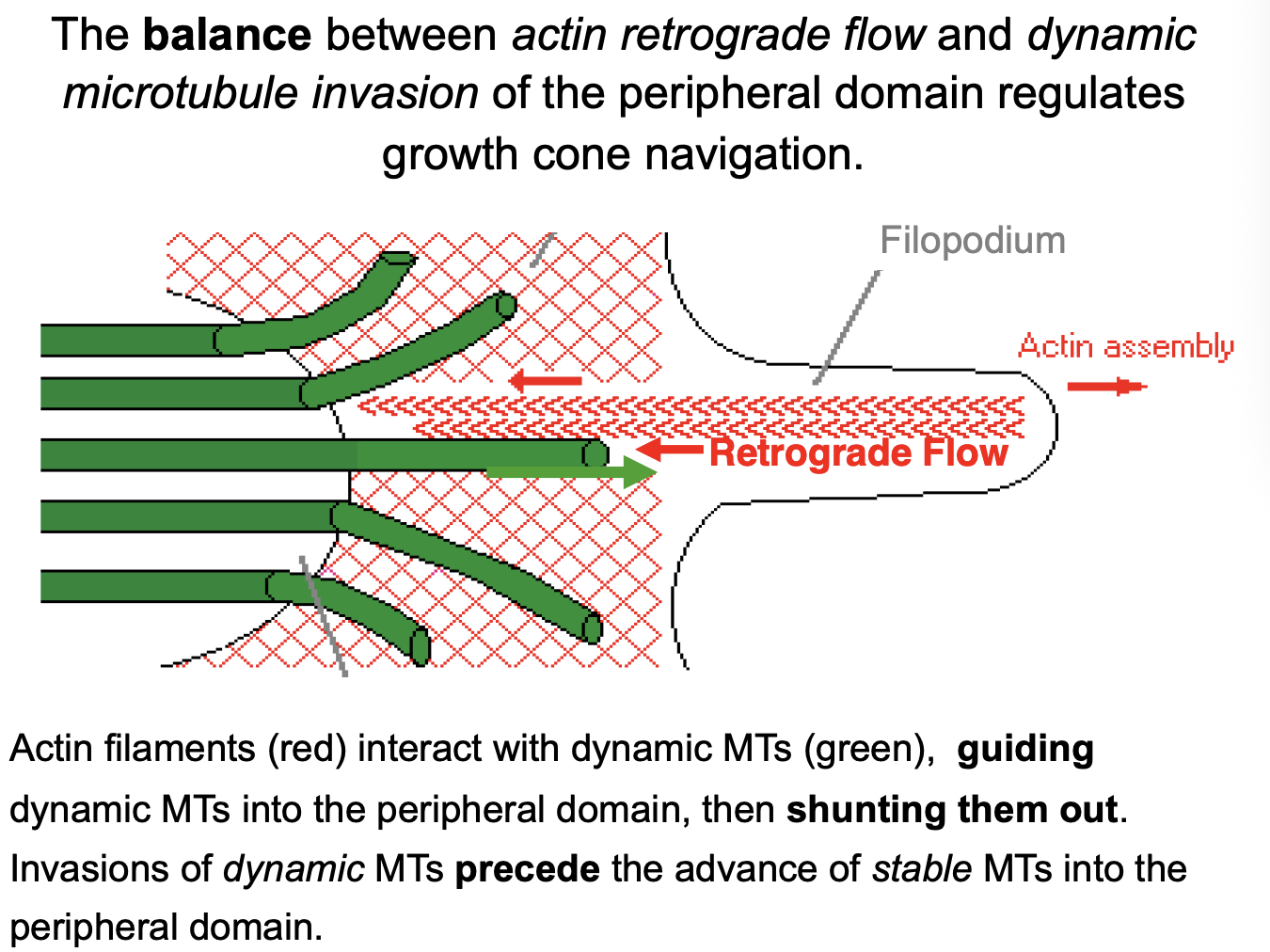
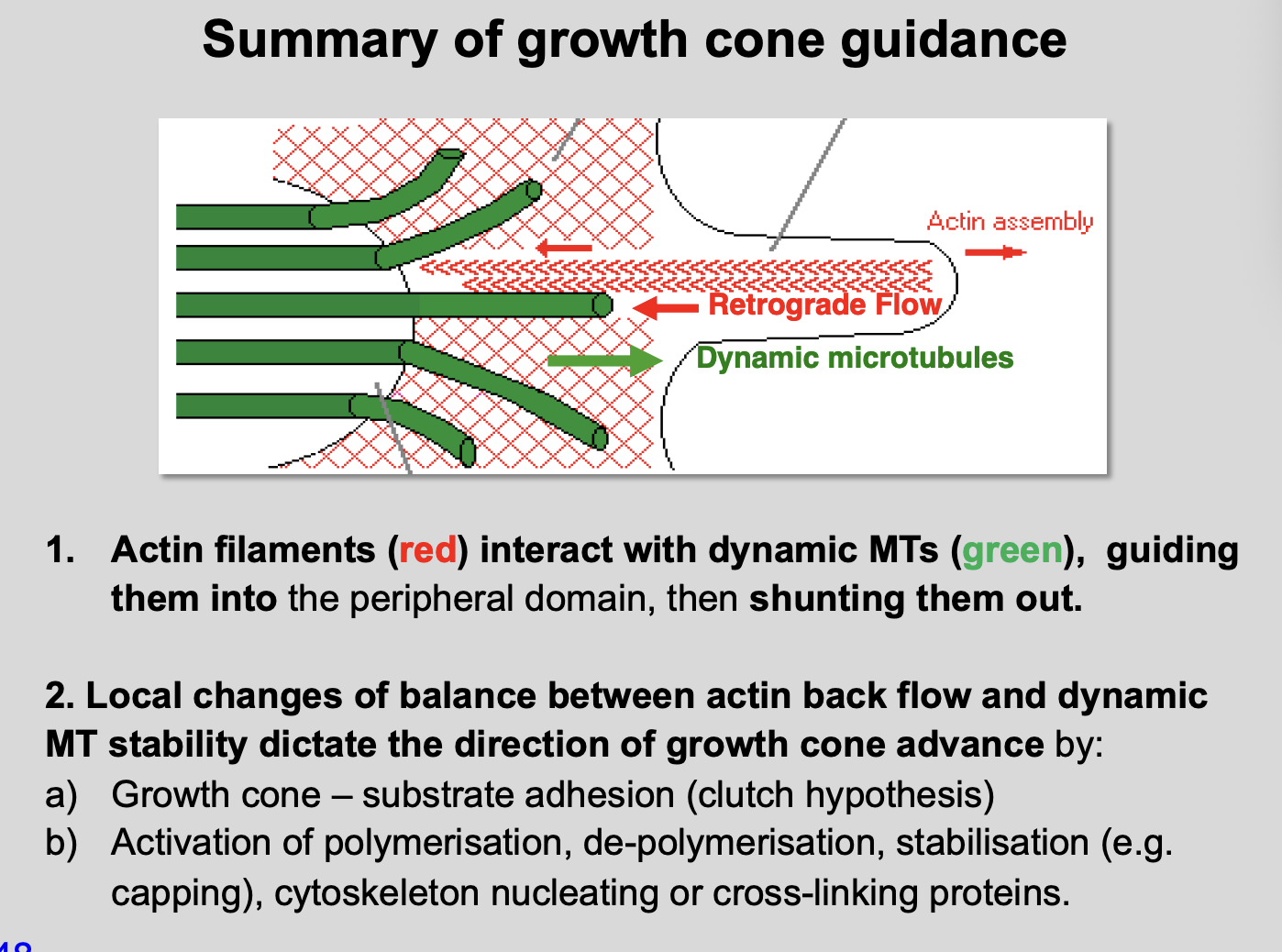
How do the actin filaments interact with dynamic MTs
interact, guiding dynamic MTs into the peripheral domain
then shunting them out
transient invasions of dynamic MTs precede the advance of stable MTs into the peripheral domain
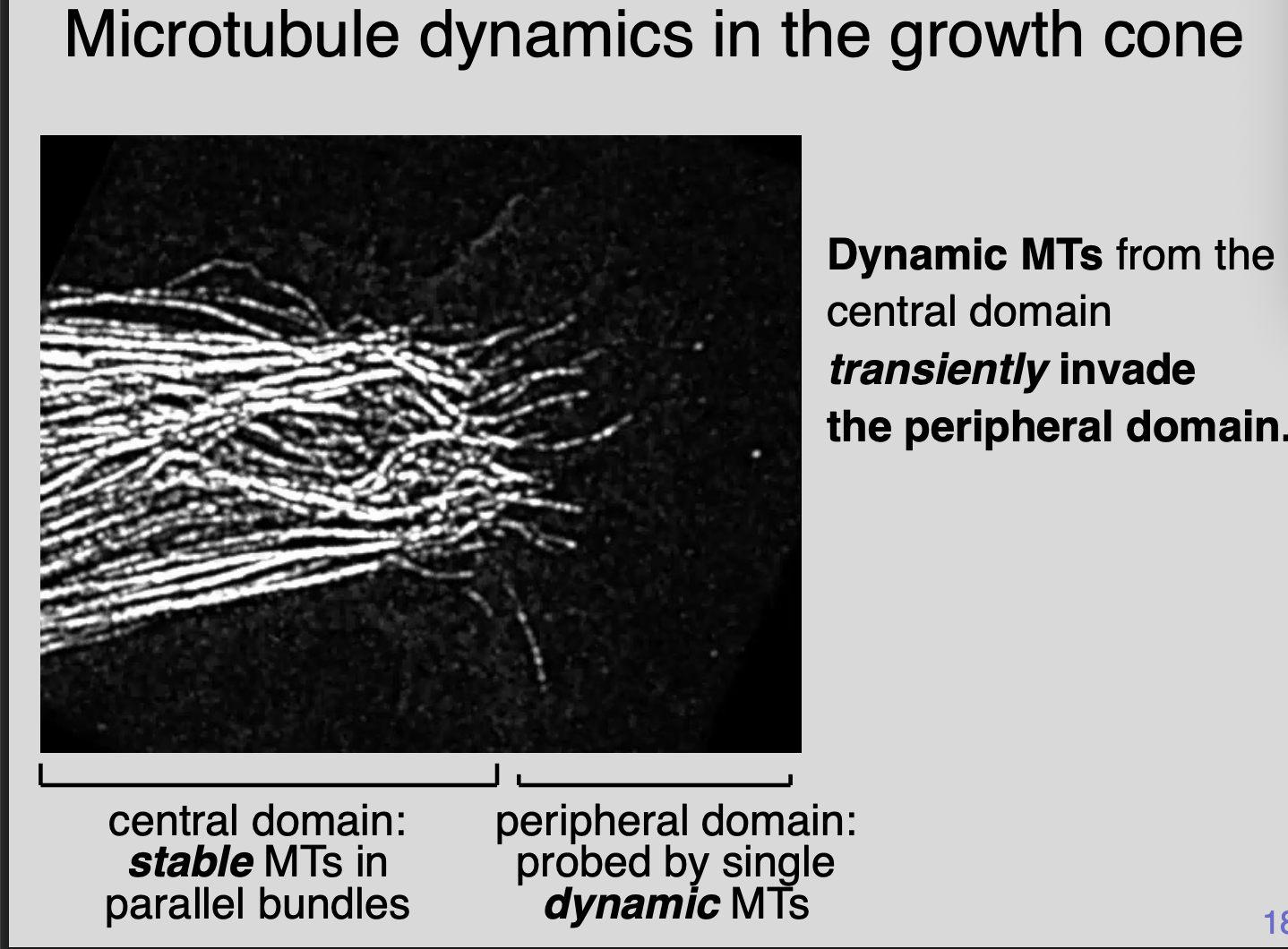
Imaging MT dynamics
Dynamic MTs from the central domain transiently invade the peripheral domain
Central domain→ stable MTs in parallel bunds
Peripheral domain→ probed by single dynamic MTs
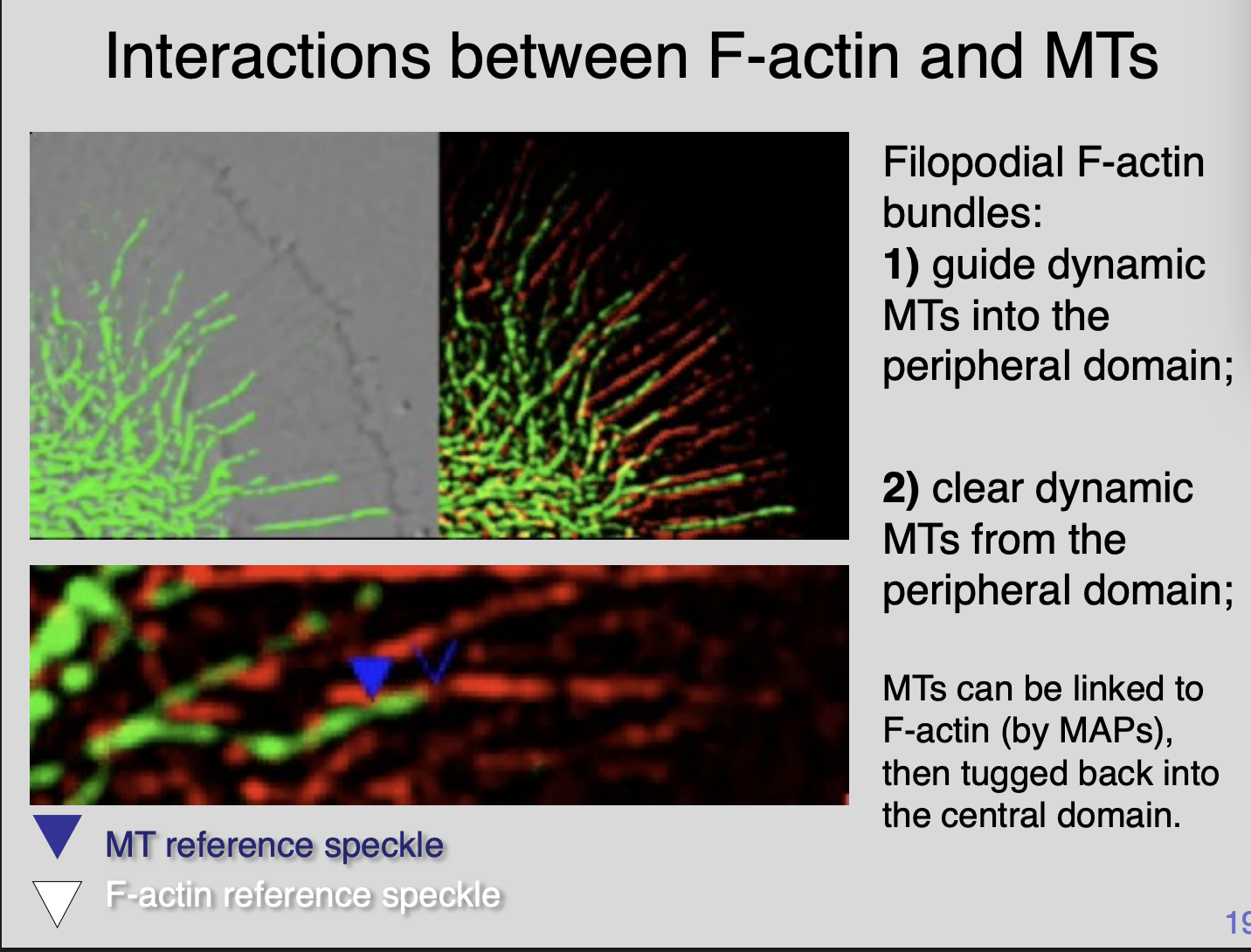
Imaging the interactions between F-actin and MTs (what do the F-actin bundles do)
Filopodial F-actin bundles
Guide dynamic MTs into peripheral domain
Clear dynamic MTs from the peripheral domain
MTs can be linked to F-actin (by MAPS)→ then tugged back into the central domain
→ can also be affected by the substrate it is on
How do growth cones achieve directed growth?
Adhesion of filopodium to a substrate via cell surface receptors and cell adhesion moelcules→ transduced to actin cytoskeleton
this decreases the myosin powered retrograde flow of F-actin
Thus decrease reate at which dynamic MTs can be shunted out of the filopodia
therefore reduced F-actin retrograde flow favours the establishemnt of MTs within the filopodium
Stabilisation of a dynamic microtubule within a filopodium promotes invasion of other microtubles
This stabilises the filopodium
thereby determines the direction of growth cone advance
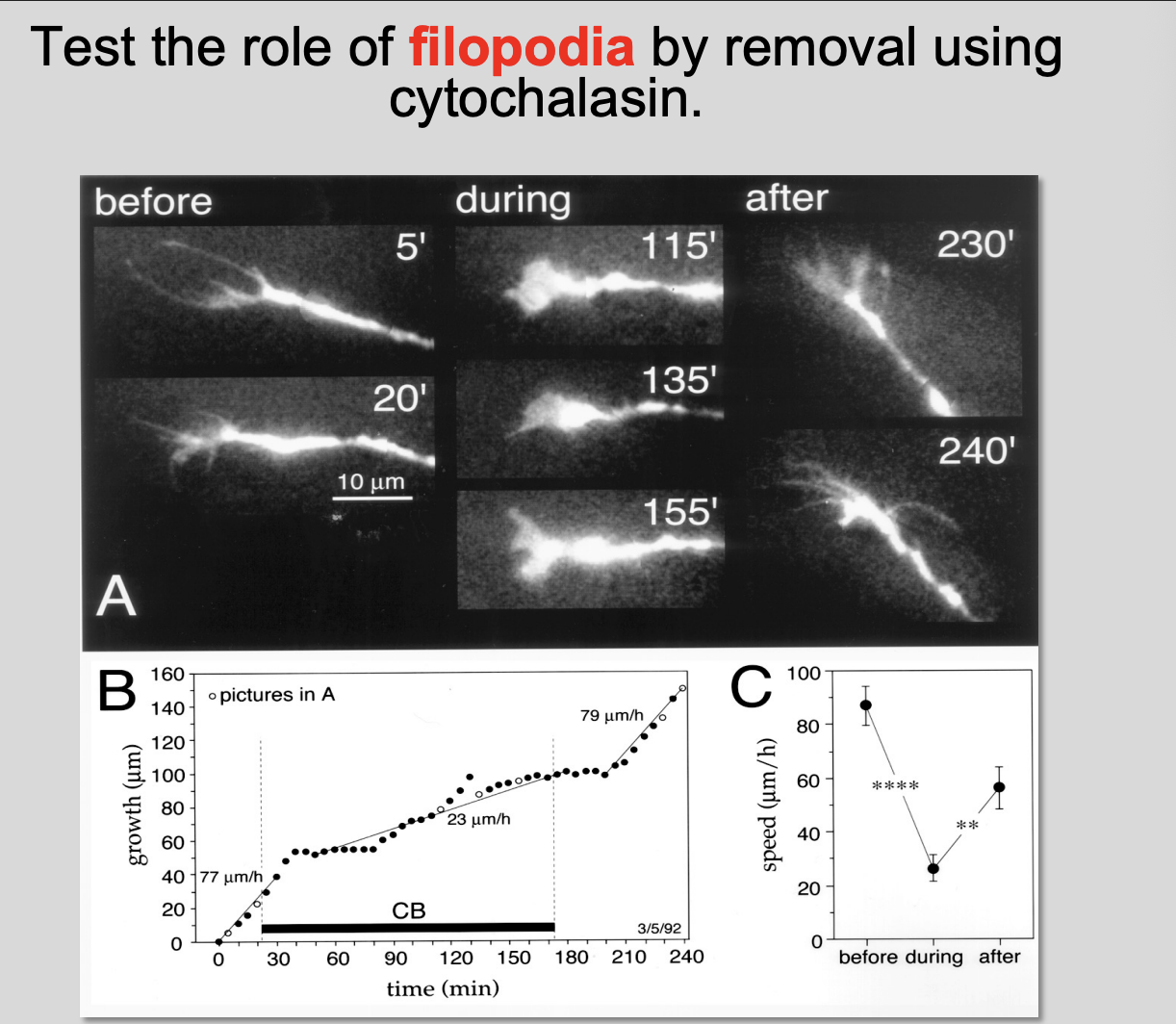
Testing the role of filopodia in the turning
Procedure
removing filopodia by using cytochalasin
find a concetration where the filopodia just disappears
Results:
Without filopodia→ aberrant pathway finding
Remove the drug→ wash away→ get direction back
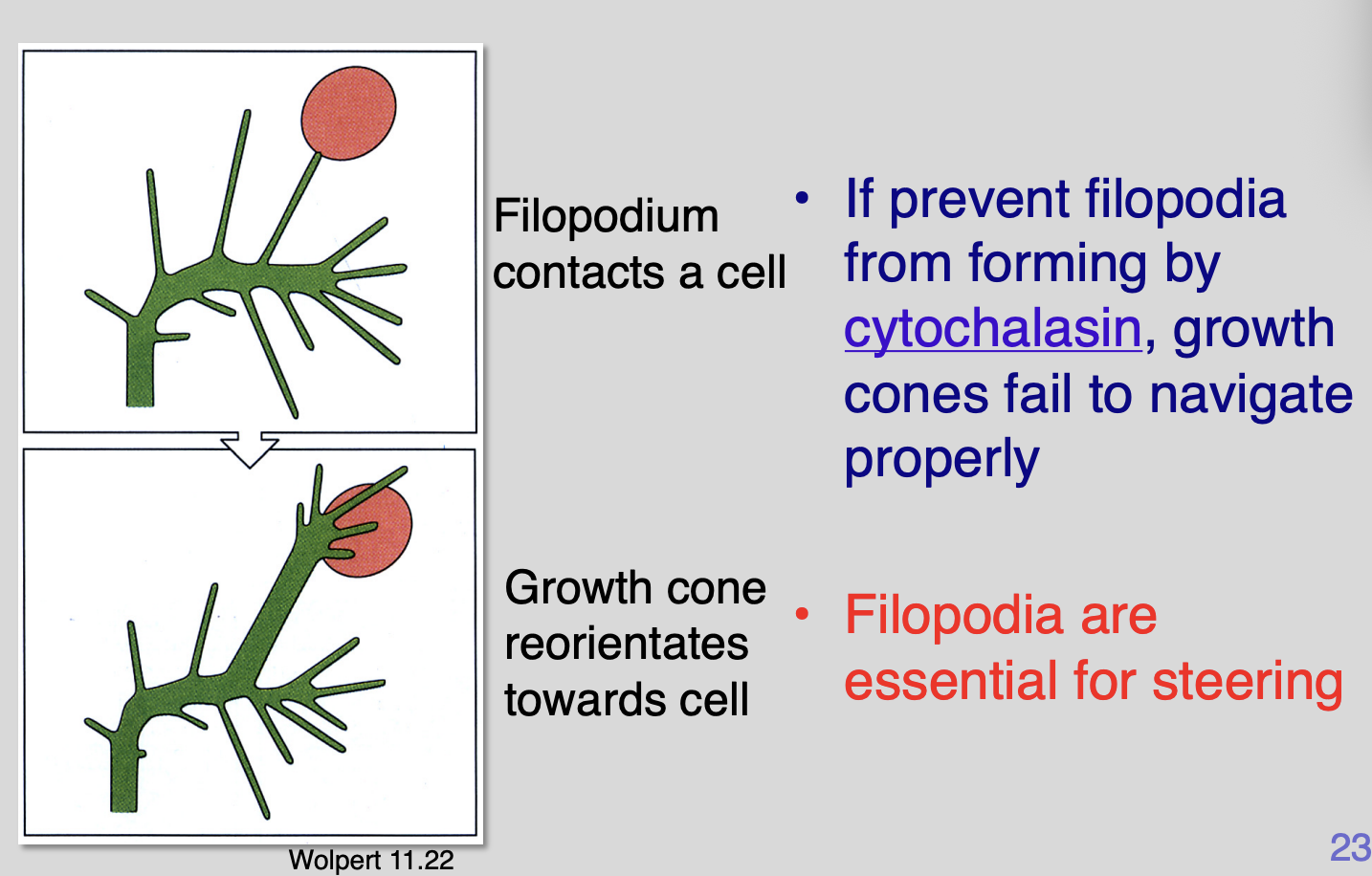
A single filopodium can direct growth
If prevent filopodia from form by cytochalasin→ growth cones fail to navigate properly
Therefore: filopodia are essential for steering
Experiments showing how Dynamic MTs determine growth cone turning
Micropiteppte experiments
control→ random growth
Taxol→ stabilises MTs→ so go twards the direction of the substance
Nocodazole→ depolymerises→ MTs are more stable on the opposite side to the substance
Overview of actin filaments and dynamic microtubules
Actin filaments in filopodia serve as tracks
Therese direct dynamic microtubules→ which themselves determine growth cone turning
Growth cone turning is regulated through changing the balance between:
Actin polymerisation vs retrograde flow and depolymerization
like non muscle myosin II motors→ seeding and serving proteins
Microtubule growth dynamics
capping proteins that stabilize vs severing proteins
Interactions between actin and microtubule cytoskeletons
like linker proteins
Any and all proteins involved in these processes are potential points for regulating growth cone guidance
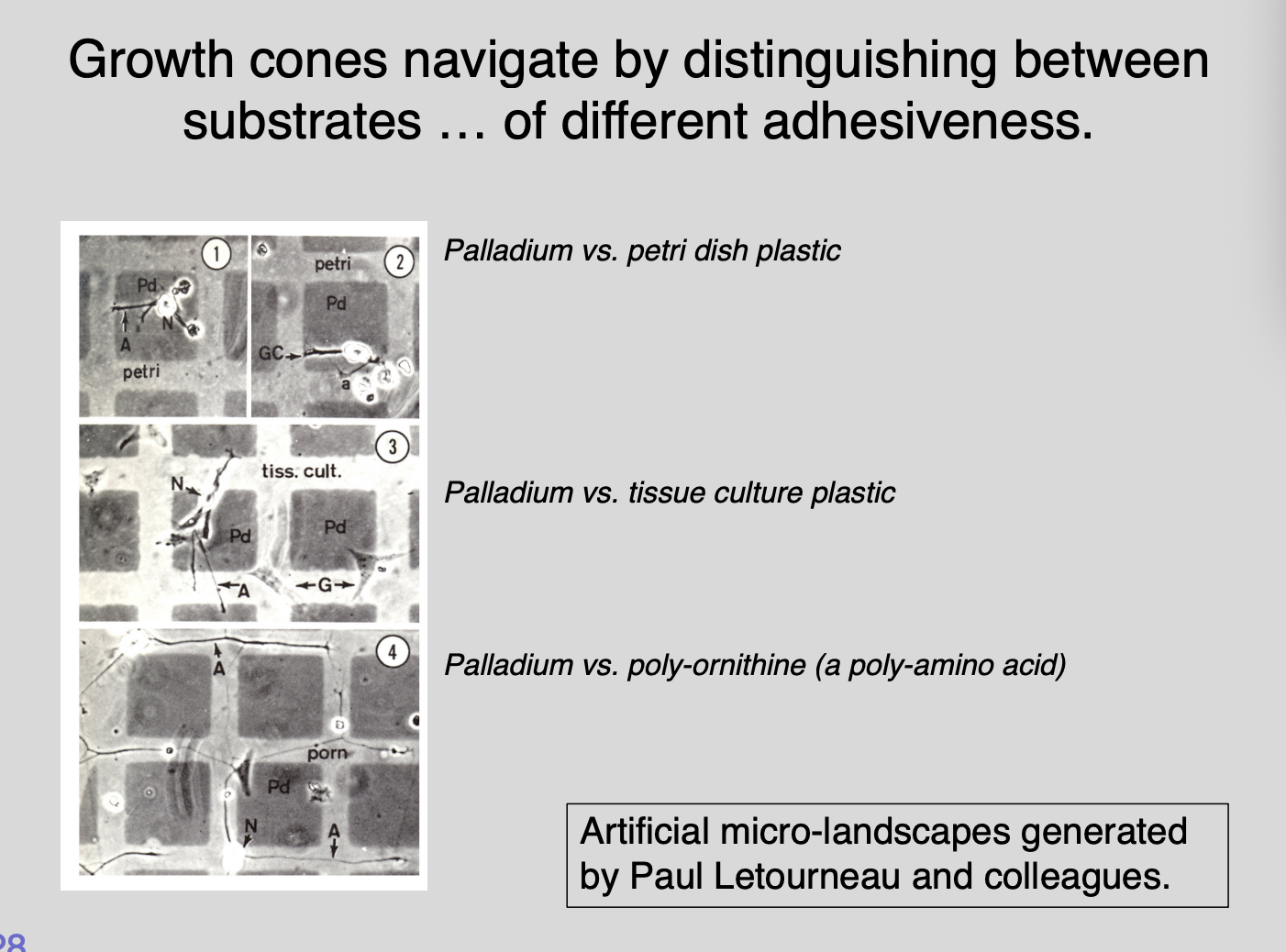
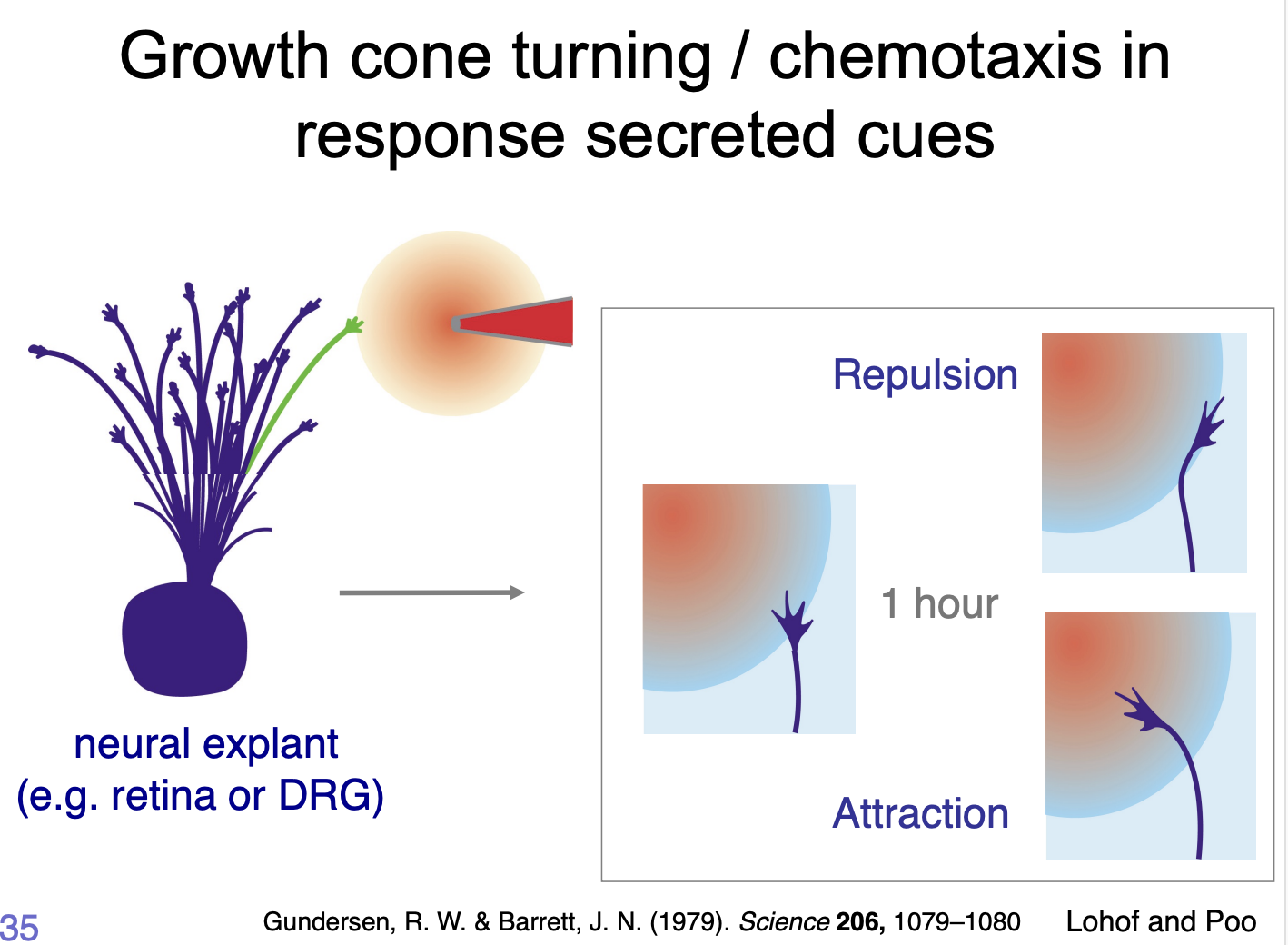
How do substrates and guidance cues direct growth cone navigation?→ Electron microscopy grid experiments Letourneau
Procedure:
coating islands in one substrate compared to others
generate artificial landscapes of differential adhesivness
have different adhesievness
test for growth
Results:
Most growth from intermediate stickiness
showed distinct preferences of growth cones to extend over some substrates but noth otherrs
What this shows→ need to be sticky enough for traction but not glued down
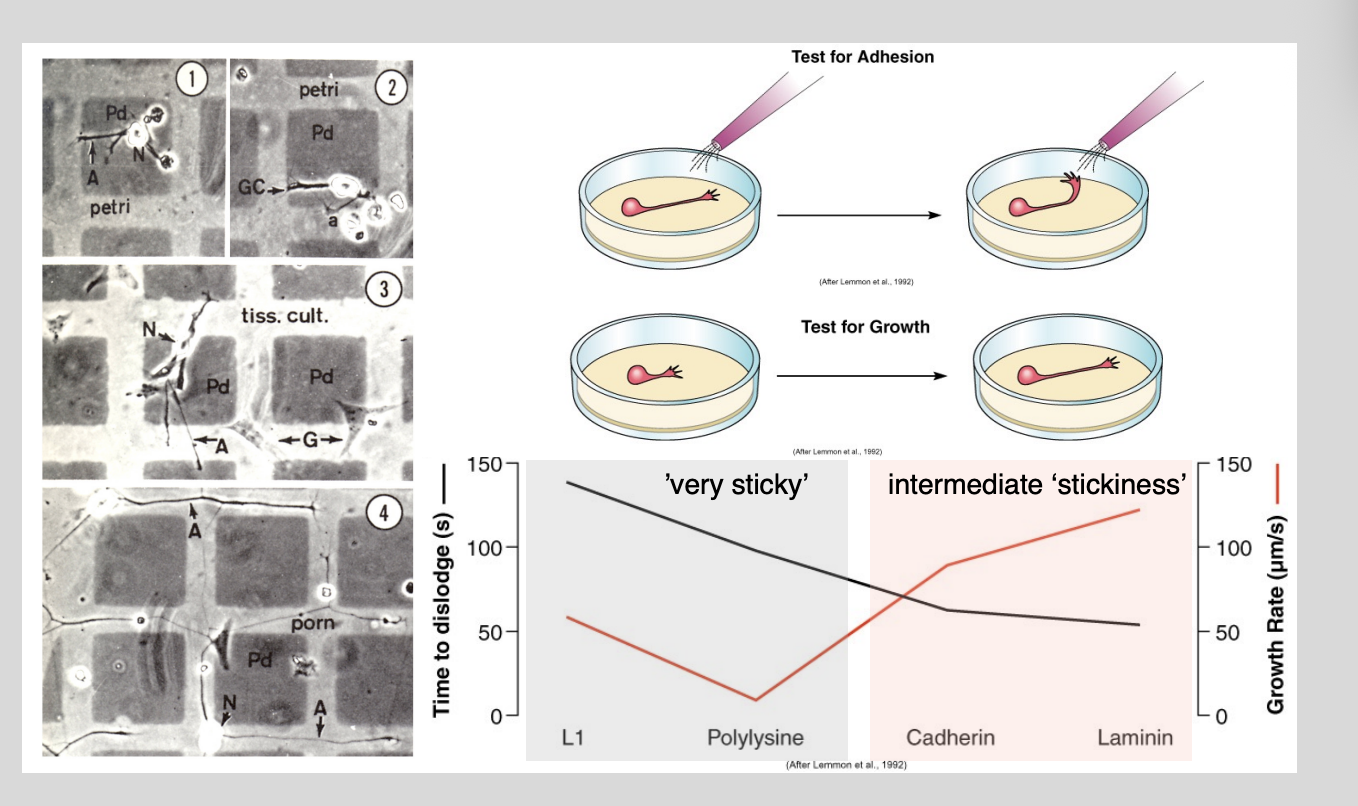
Results found for Concanavalin-A (most adhesive)
Most adhesive substrates
BUT
in fact poor supporters of axonal growth
seem to glue axons down to the extent of immobolising them
We now know that→ such adhesive landscapes also exist in the developing embryo
How do these interactions work in vivo?
Combinations of receptors confer substrate choices→ Form a ‘molecular clutch’
Components remain incompletely characterised
Extracellular matrix proteins (ECM)→ (substrate)
e.g Lamin and fibronectin and various collagens
→ promote axon outgrowth
Intracellular→ integrin receptors (alpha and beta subunits)
particular neuron expresses at a given developmetnal stage
differen ECM molecule preference
types of substances it interacts with depends on the types of receptors it can interact with
These interactions can change in development
e.g chick retinal ganglion cell axons:
alpha-6 integrin subunit→ prefer lamin as a substrate over fibronectin
BUT→ with maturation of alpha-6 expression
what?????
Integrins can also interact with the neuronal guidance cues
Netrins and Semaphorins
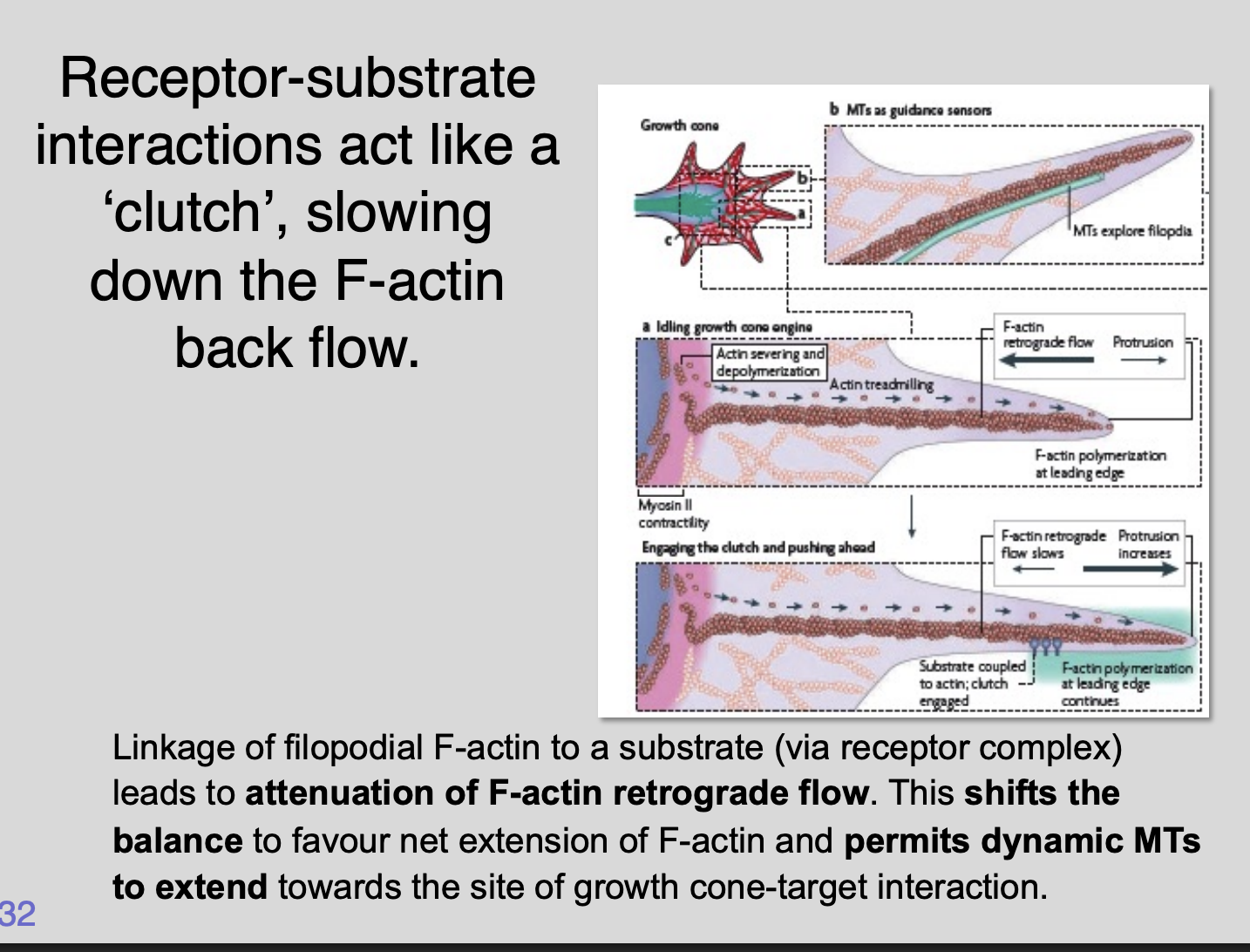
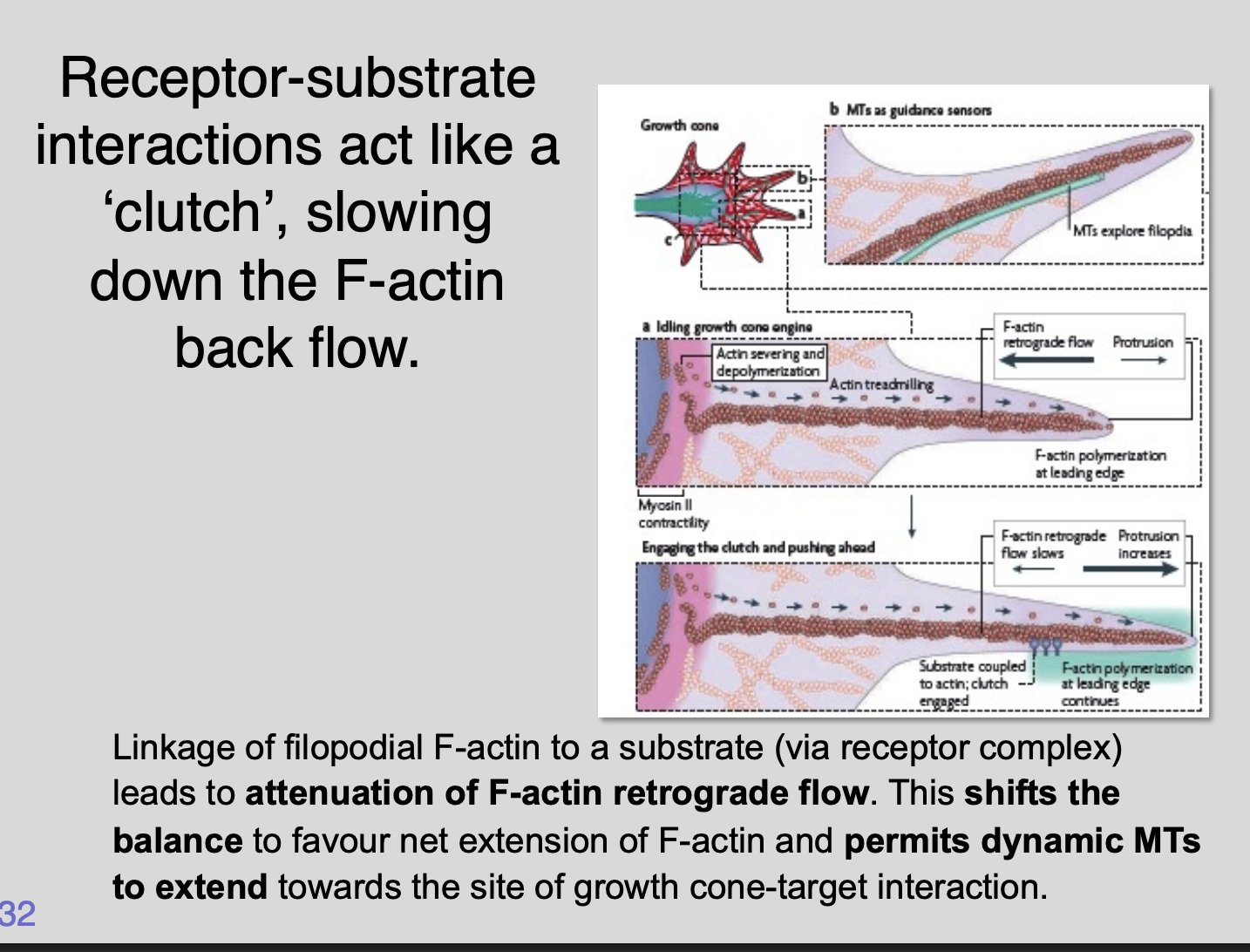
How do interactions with extracellular substrates change the dynamics of F-actin and microtubules inside the growth cone
Integrin receptor complexes form a direct link betweenreceptor
-substrate interactions act like a clutch
slowing down the F-actin back flow
Linkage of filopodial F-actin to a substrate (via receptor complex)
lead to attenuation of F-actin retrograde flow
Shifts the balance to favour net extension of F-actin
Permits dynamic MTs to extend towards the sit of growth cone-target interaction
Examples of CAMs
Some have been found to interact directly with the cytoskeleton
e.g Neural Cell Adhesion Moelcule (NCAM) isofroms 140 and 180 are associated with alpha-actinin and tubulins
The numerous proteins nucleate, stbilise fragment, cap and crosslink actin filaments and microtubules
the activities and localisation of these proteins can be regulated through receptors on the growth cone surface
Other guidance cues come in the form of
Cadherins
many cell adhesion molecules (CAMs) largely of the immunoglobulin superfamily
e.g N-CAM
some mediate homophilic
some mediate heterophilic interactions
Overall Ascepts of growth gruidance
Differential adhesiveness
Guidance cues that function as signals
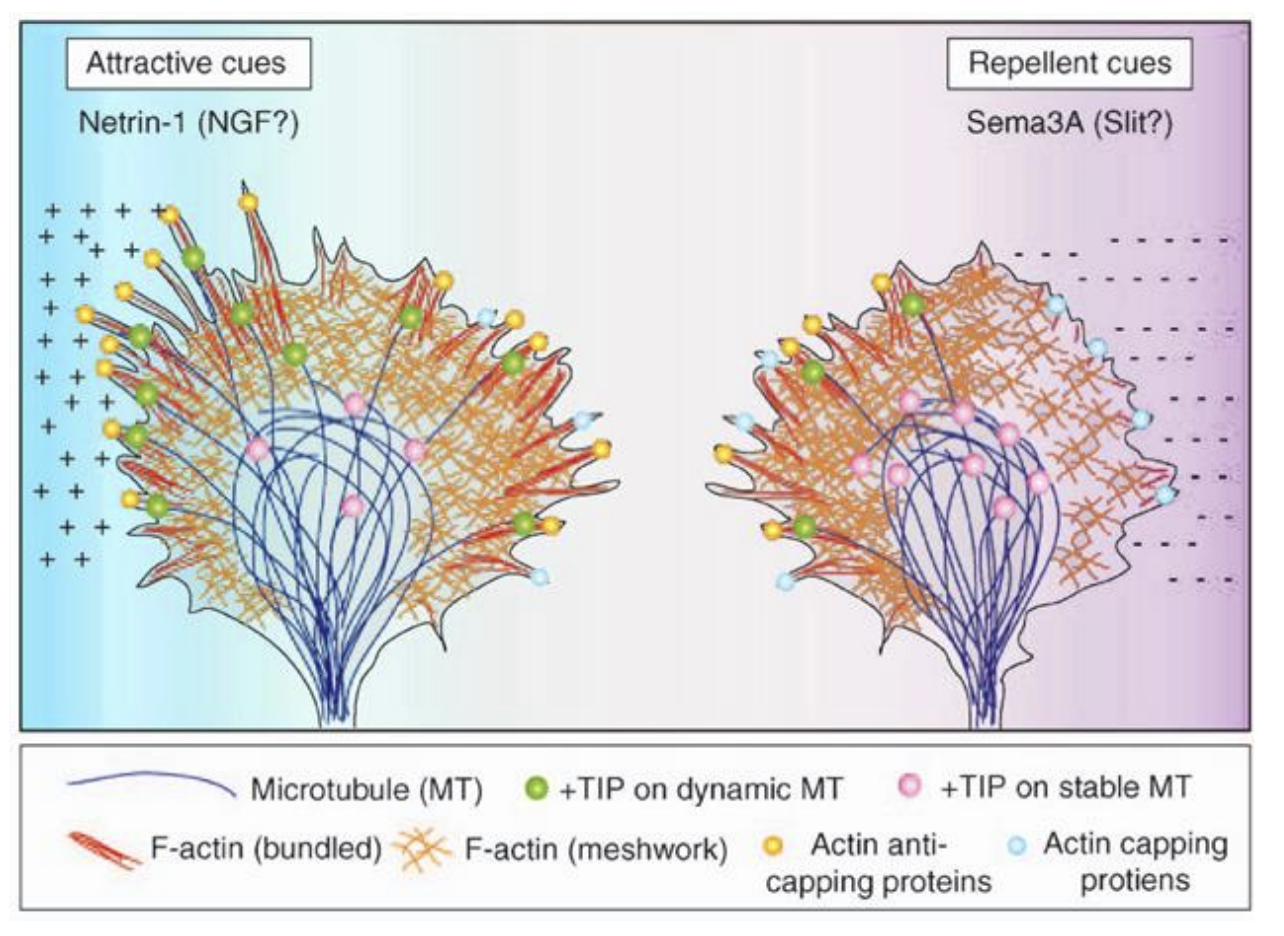
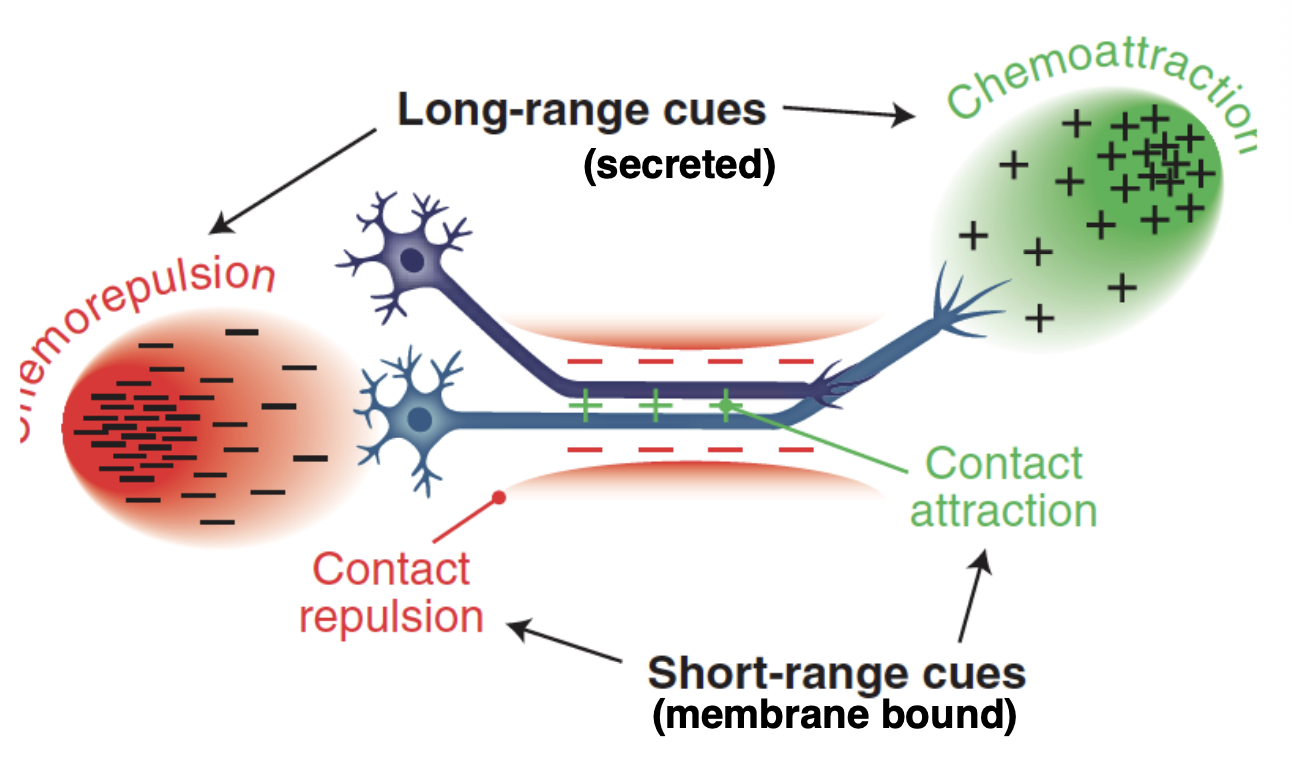
What is axon guidance controlled by
Long range cues→ secreted (soluble)
Short range cues→ membrane bound (physical attachment) immobilised
What do these cues do
trigger an intracelular signalling cascade that modify the cytoskeletal dynamics
causing
advance→ attractive
collapse/retraction→ repulsive cues
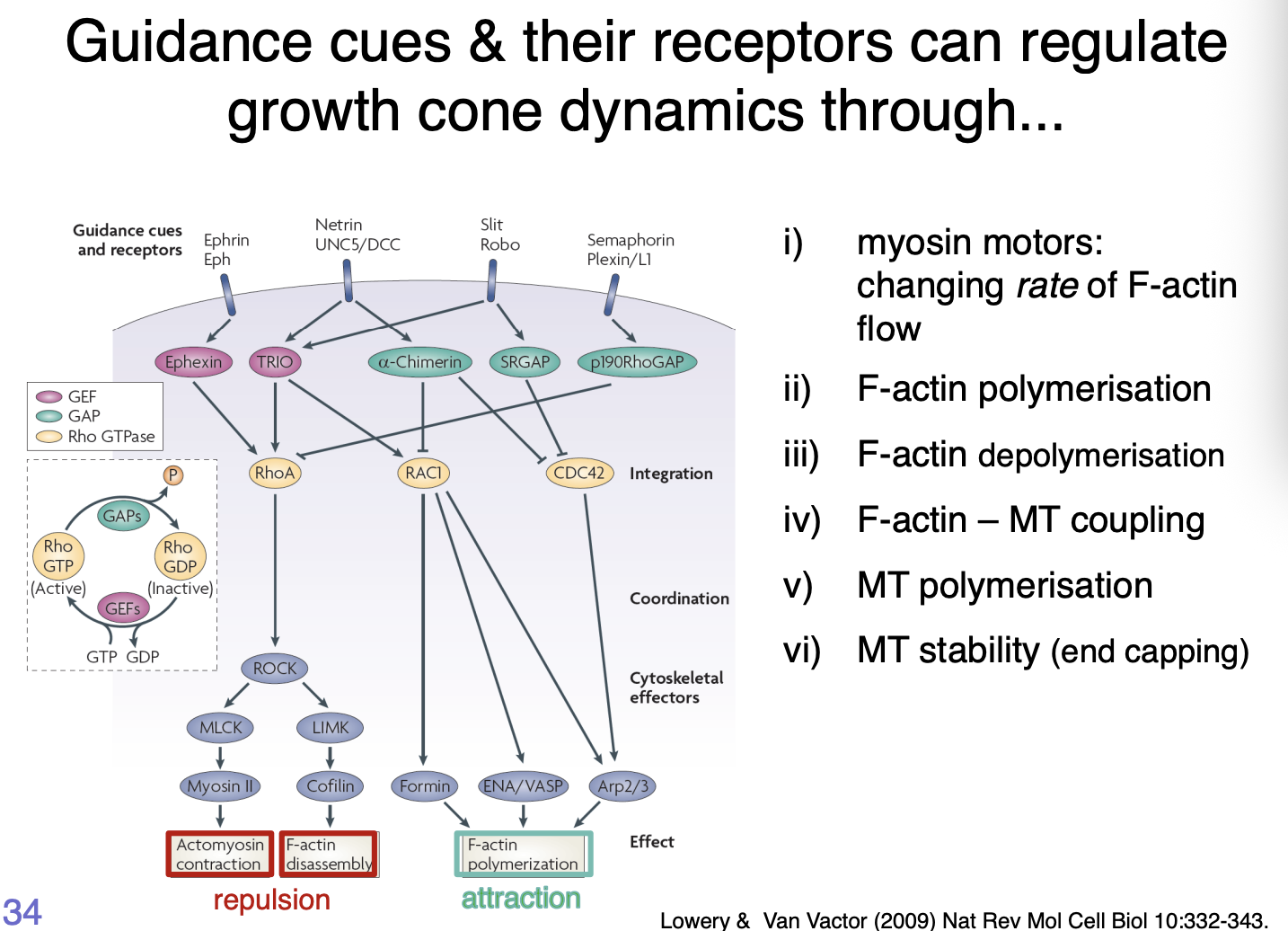
How do guidance cues and their receptors regulate growth cone dynamics
Receptors to the Slit, Netrin, Ephrin and Semaphorin families of guidance cues signal through intracellular pathways
Part of these are the Rho family of small GTPases
act as integrators of multiple signalling pathways
Attractive response → activation of the small Rho family GTPases Rac and CDC42 →Promotes actin polymerisation
Repulsive cues→ activate RhoA→ decrease actin polymerisation
Summary of how cues work
Myosin motors→ changing rate of F-actin flow
F-actin polymerisation
F-actin depolymerisation
F-actin- MT coupling
MT polymerisation
MT stability (end capping)
note: robo here see next lecture
Some cues can be attractive or repulsive→ depending on the context example
Netrin signalling through the netrin receptor DCC
Generally→ attraction
Addition of Netrin co-receptor Unc-5→ repulsion
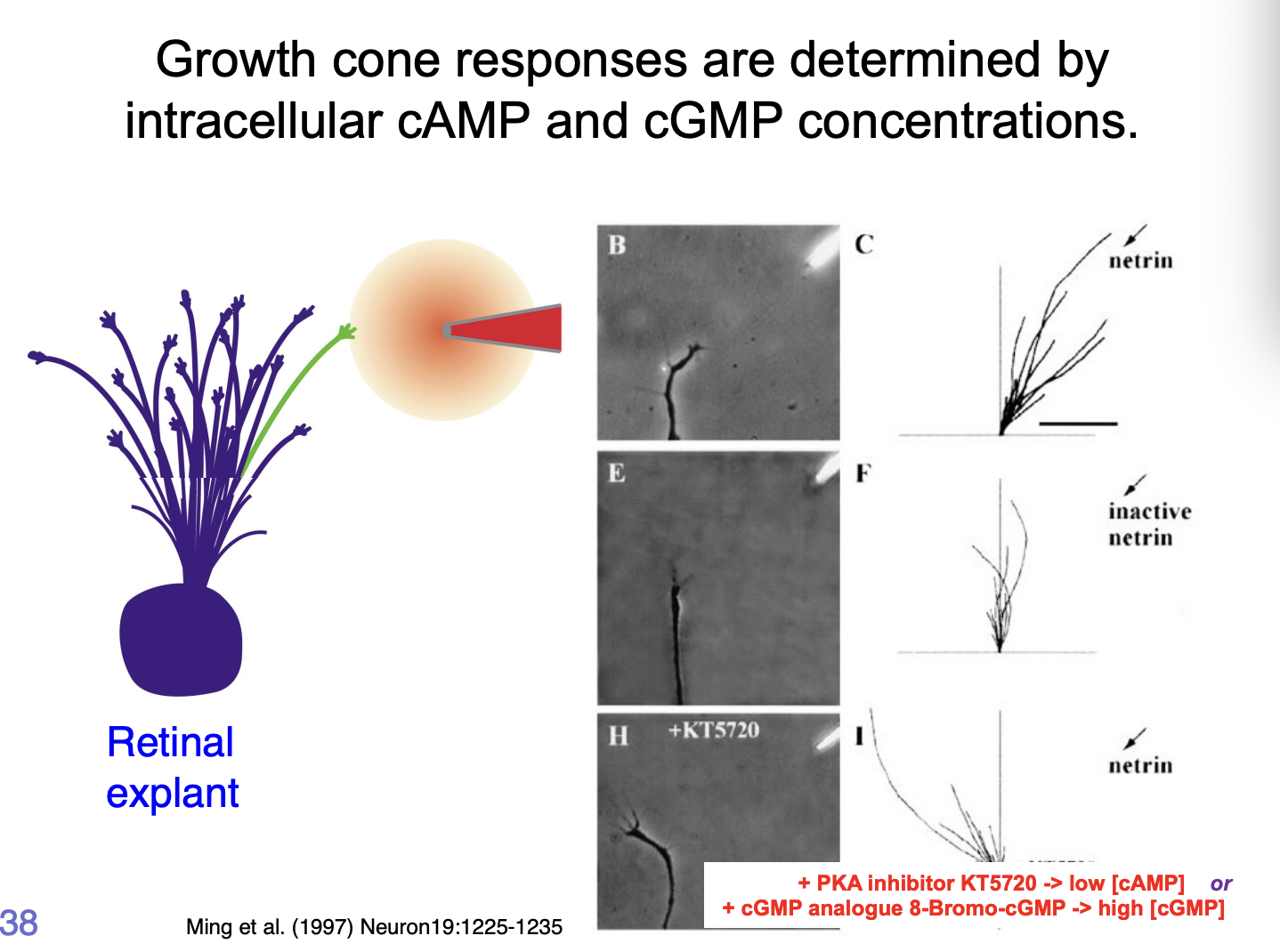
What are the growth cone responses to these cues mediated by
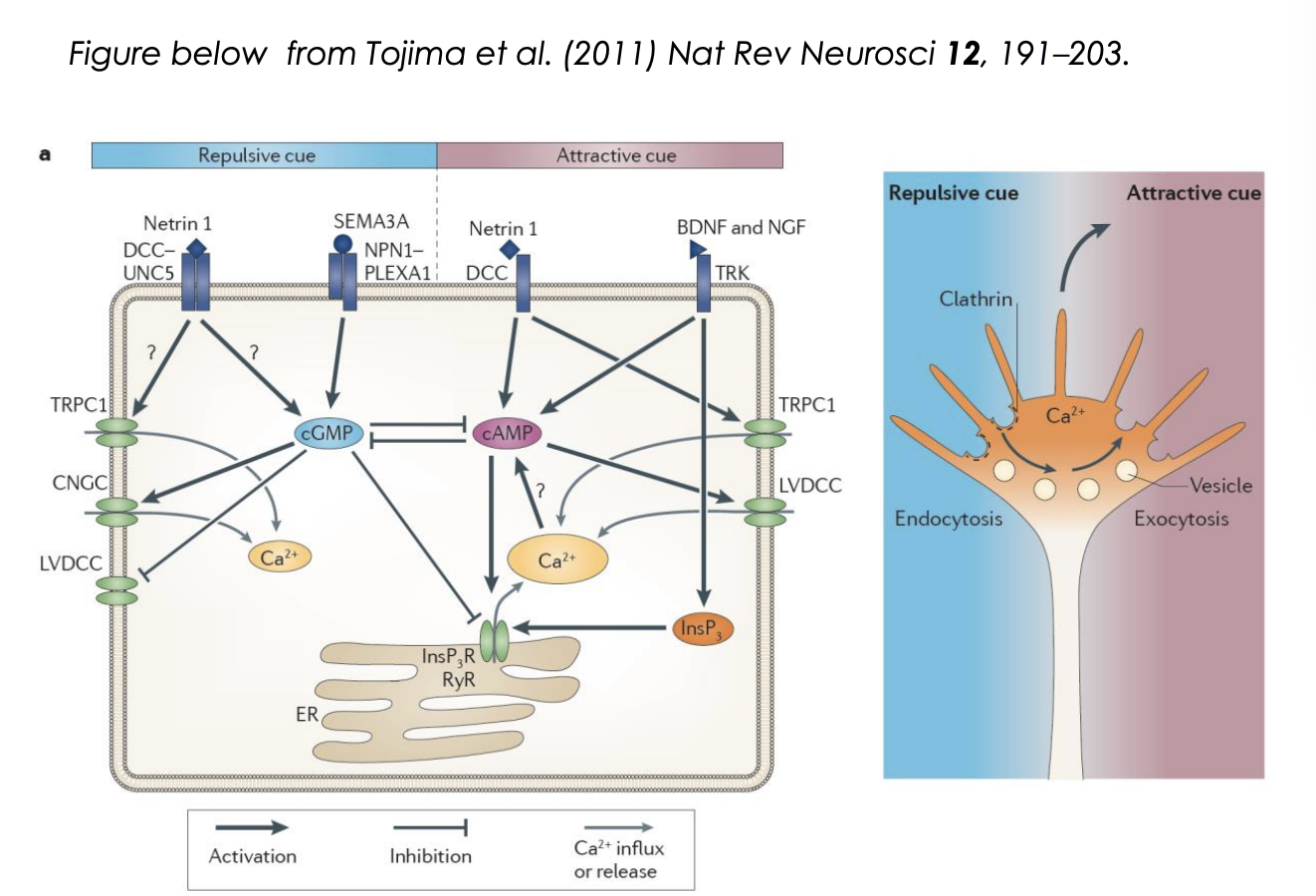
Intracellular [cGMP] : [cAMP]
High [cAMP]/[cGMP]→ attraction
Low [cAMP]/[cGMP]→ repulsion
How makes netrin repulsive vs attractive at certain points:
due to activity of kinases (PKA)
if PKA is inhibited→ changes the ratio of cGMP to cAMP
![<p><strong>Intracellular [cGMP] : [cAMP]</strong></p><ul><li><p>High [cAMP]/[cGMP]→ <strong>attraction</strong> </p></li><li><p>Low [cAMP]/[cGMP]→ <strong>repulsion</strong></p></li></ul><p></p><p><em>How makes netrin repulsive vs attractive at certain points:</em></p><ul><li><p>due to activity of kinases (PKA)</p></li><li><p>if PKA is inhibited→ changes the ratio of cGMP to cAMP</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/2fcb6f74-6ee1-4dcd-ad3d-a1a7a975c970.png)
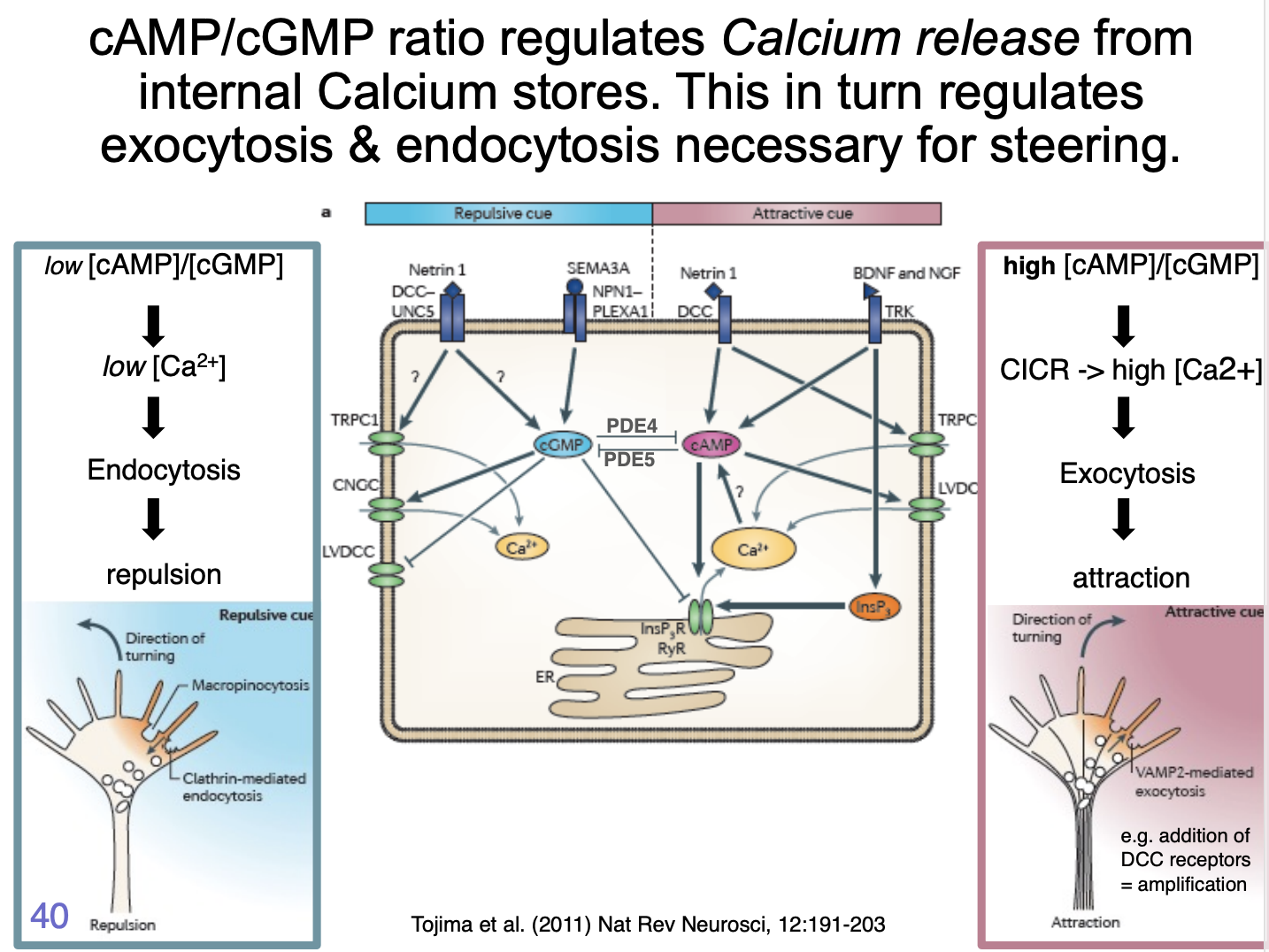
How does the cAMP/cGMP ratio regulate the response? Attraction
It regulates Calcium release from internal calcium stores
Attraction:
cue triggers influx of extracellular calcium through L-type calcium channels
Ca2+ signal is amplified by calcium induced calcium release (CICR) from ER
via ryanodine receptors (RyR) or InsP3 receptors
Generates high amplitude local clacium signals
activate Ca2+/ calmodulin-dependent kinase CamKII
CamKII can phosphoryalte microtuble motors
initiating directed vesciel exocytosis at the side of elevated calcium
provides plasma membrane components necessary for growth cone tunring toward an attractive cue (so can stretch)
as well as directed deliverly of signalling and adhesion complex components
in parallel, cytoskeletal dynamics are changed to favour stabilisation and polymerisation of microtubules and filamentous actin
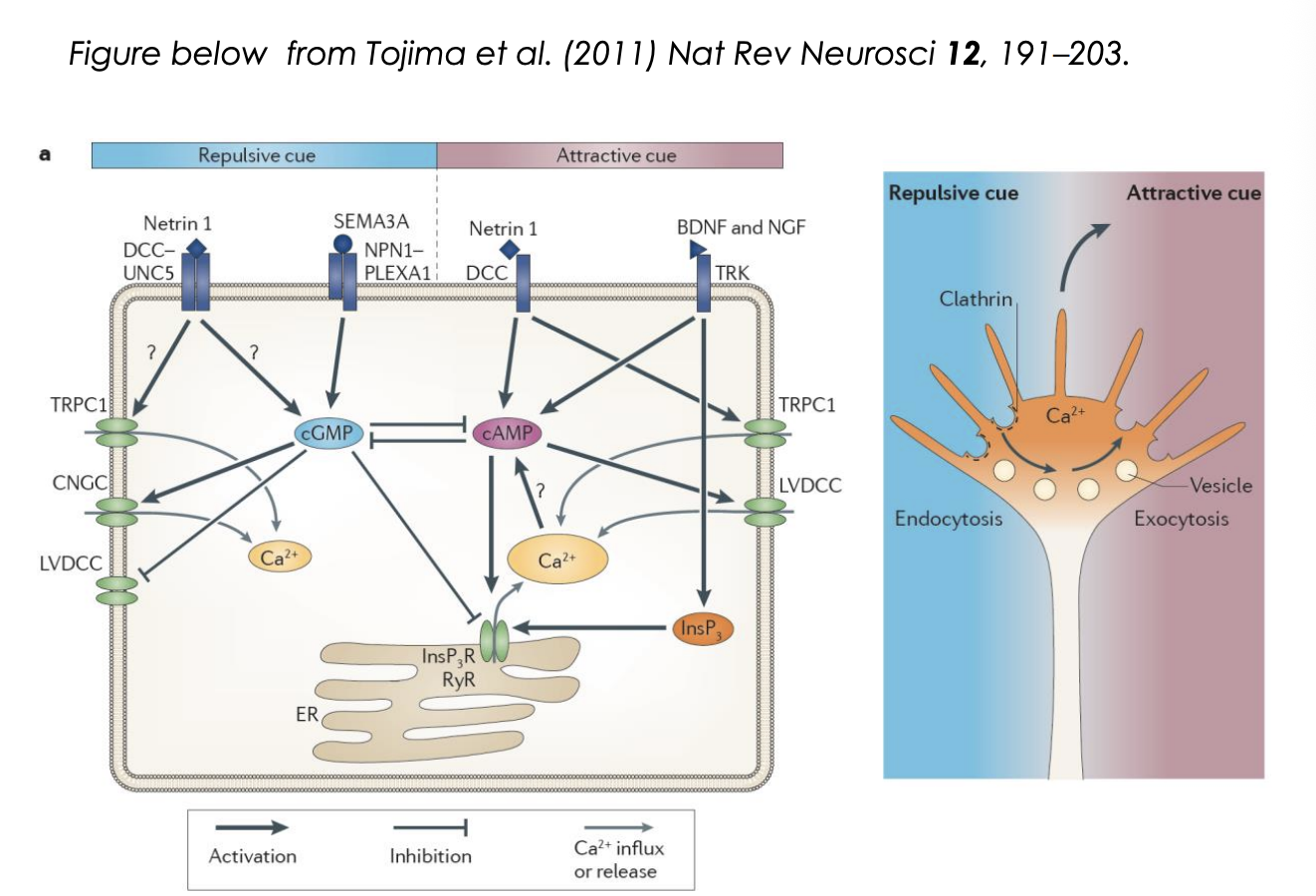
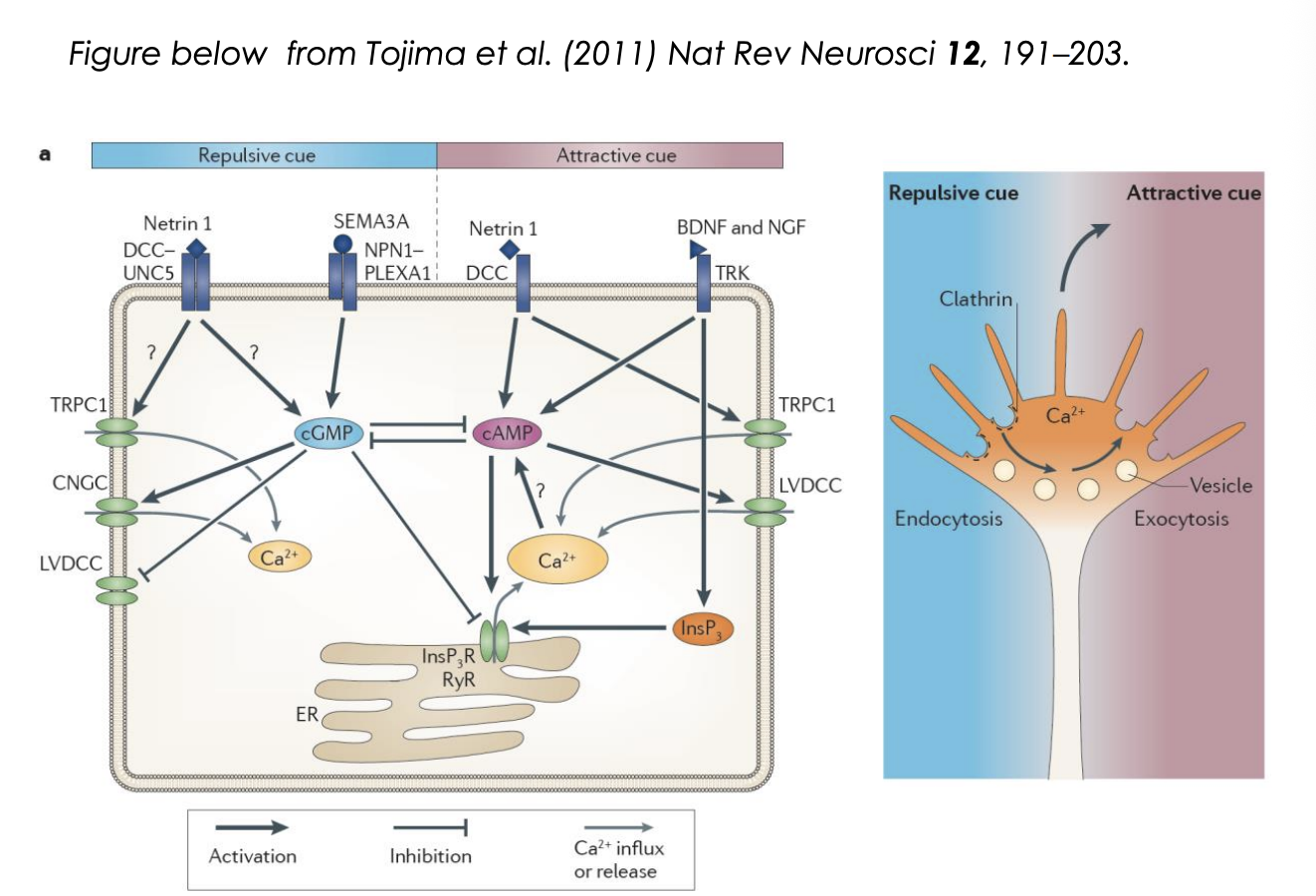
How does the cAMP/cGMP ratio regulate the response? Repulsion
repulsive cue triggers low amplitude calcium influx
not amplified by internal stores
low ampltitude signals appear to act via Ca2+/calmodulin-dependent phosphatase, Calcineurin
This has a higher affinity to Ca2+ than CamKII→ so can be activated at lower [Ca2+]
Calcineruin activation triggers clathrin mediated endocytosis
leads to growth cone retraction
partly by removal of adhesion complex components such as integrins
![<ol><li><p>repulsive cue triggers low amplitude calcium influx</p></li><li><p>not amplified by internal stores</p></li><li><p>low ampltitude signals appear to act via <strong>Ca2+/calmodulin-dependent phosphatase, Calcineurin</strong></p></li><li><p>This has a higher affinity to Ca2+ than CamKII→ so can be activated at lower [Ca2+]</p></li><li><p>Calcineruin <strong>activation</strong> triggers <strong>clathrin</strong> mediated endocytosis</p></li><li><p>leads to growth cone <strong>retraction</strong></p><ul><li><p>partly by removal of adhesion complex components such as integrins</p></li></ul></li></ol><p></p>](https://knowt-user-attachments.s3.amazonaws.com/00f3a177-fea9-4c67-a542-d6fd6d6f13b7.png)
What is decicevness facilitated by
mutual inhibition between cGMP and cAMP signalling
cGMP→ inhibits
cAMP→ promotes calcium influx
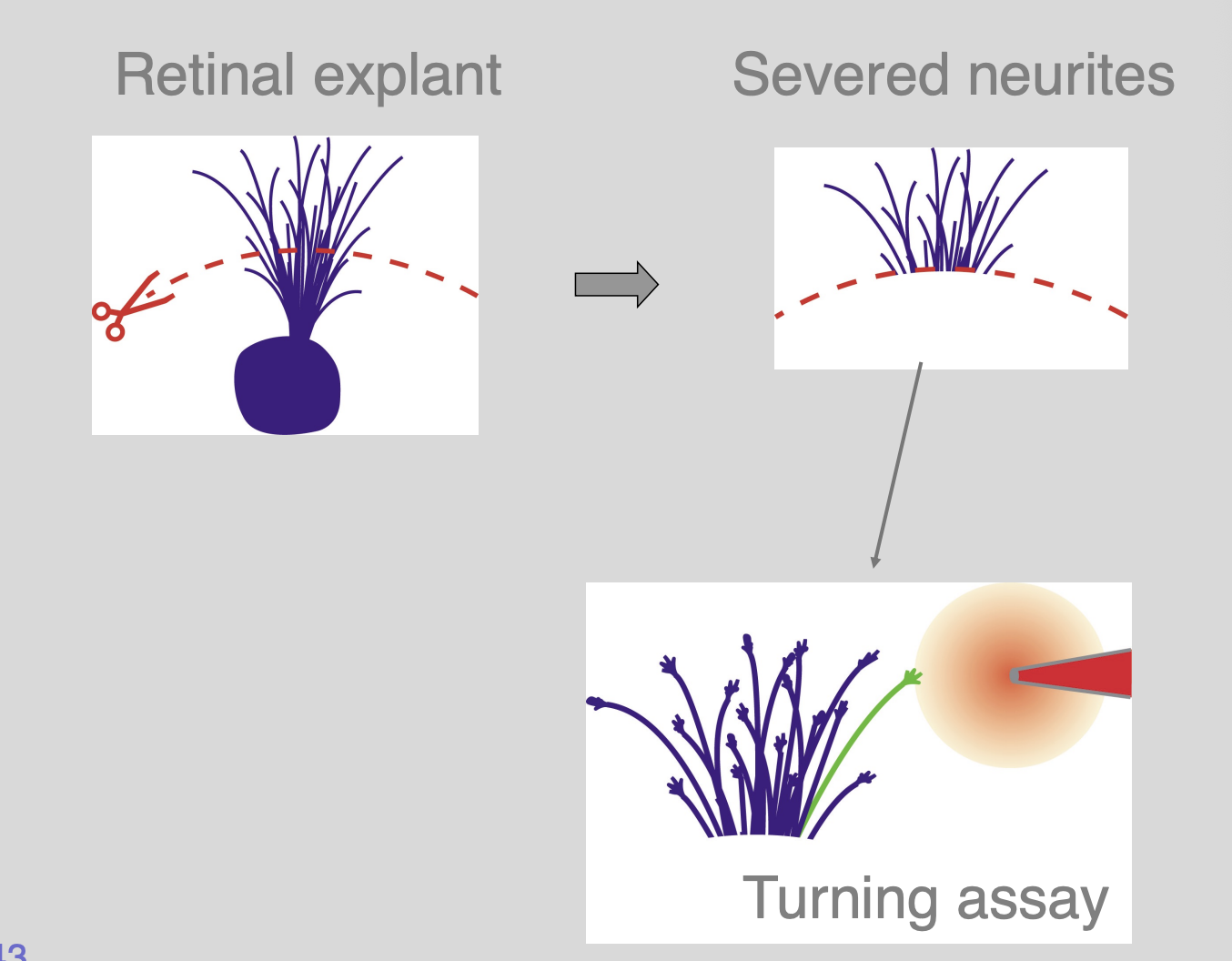
Because growth cones can navigate even if cut off from cell body this show
growth cones can navigate autonomously
What is the machinery that is necessary for pathfinding and how is it regulated?
Is transciption required?
Cut off cell body
result→ still navigate
shows→ Do not need transciption
Is protein synthesis required?
add protein synthesis inhibitors (cycloheximide, anisomycin)
result→ unbiased no direction
shows→ protein synthesis is required
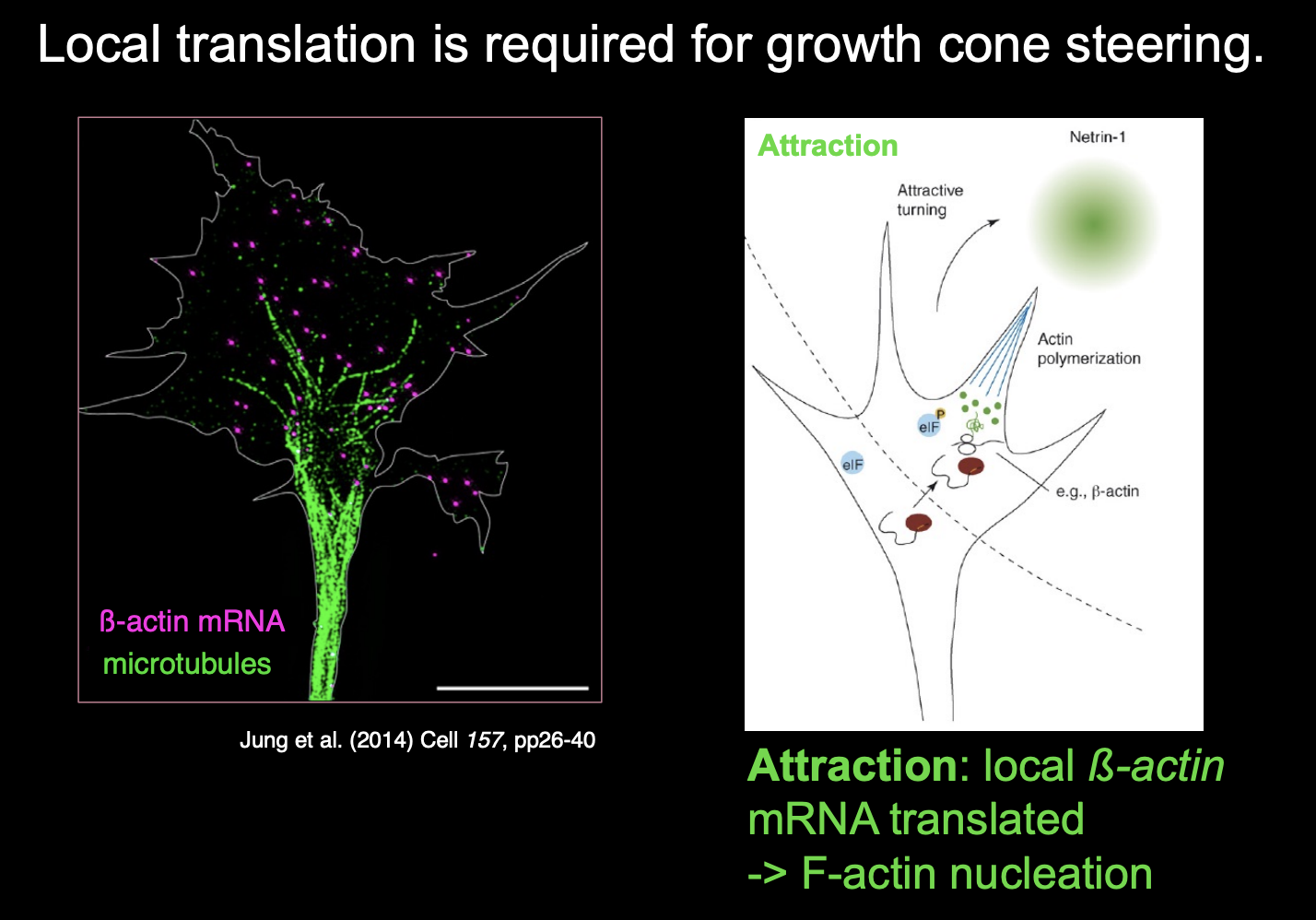
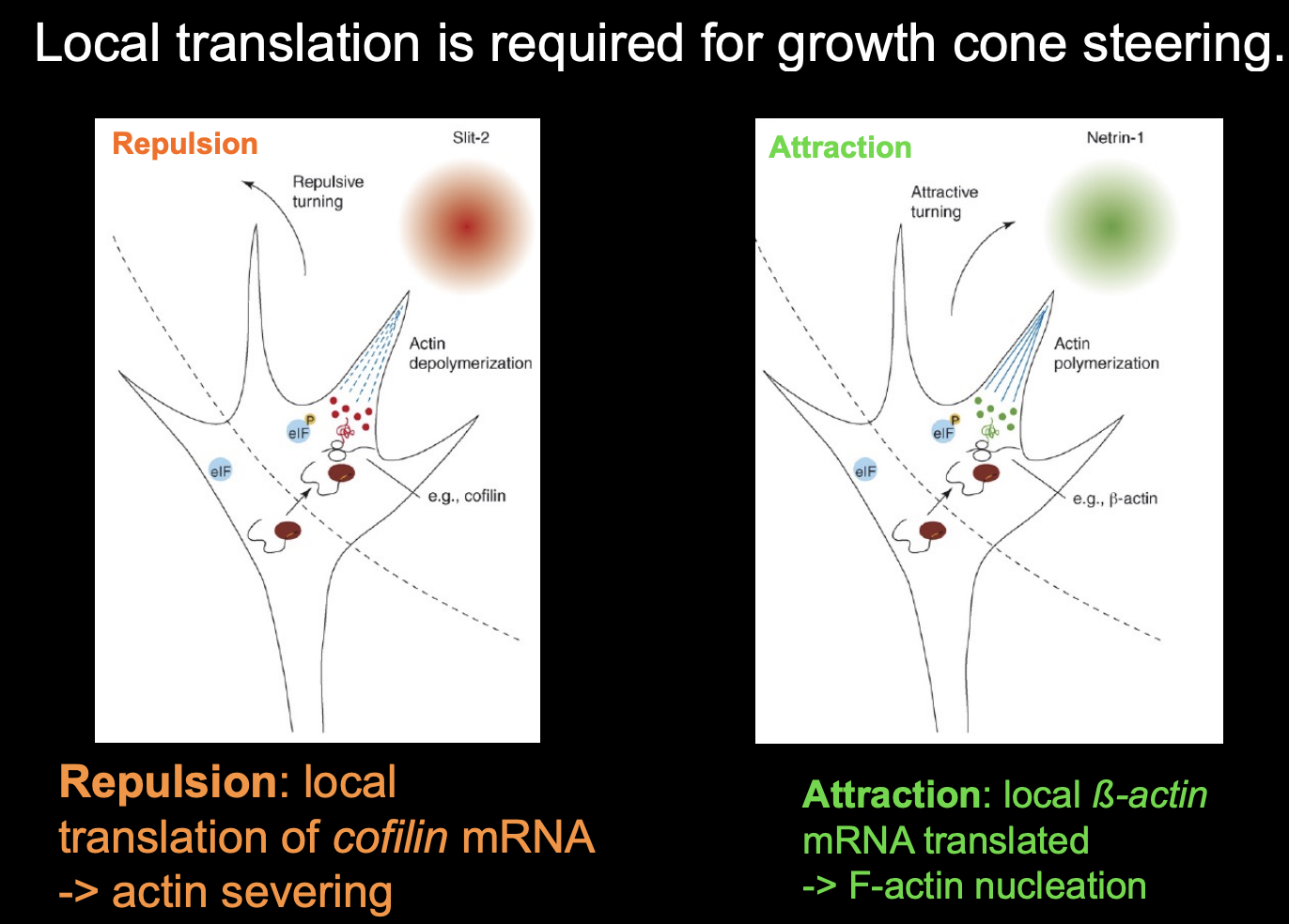
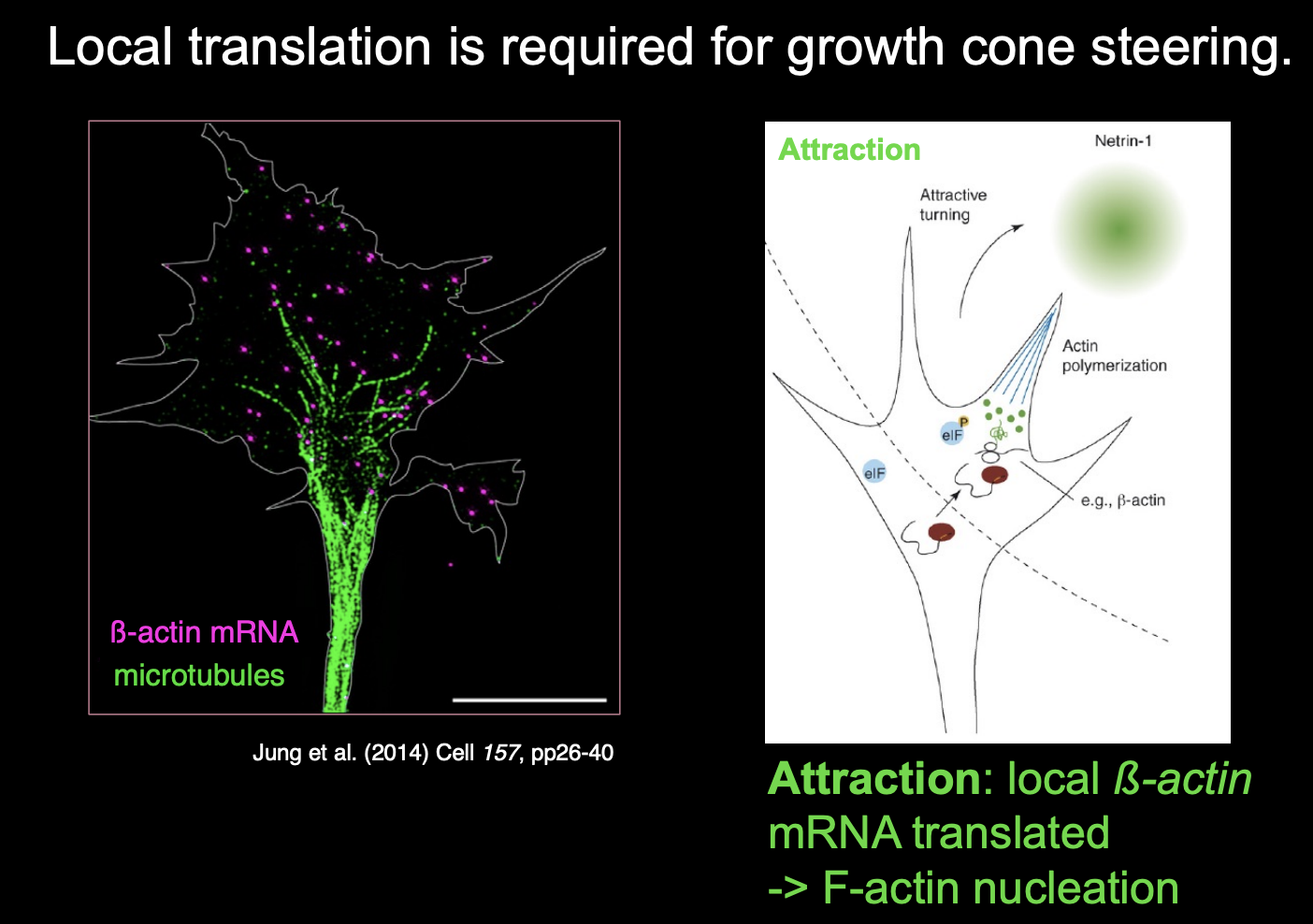
Local translation is required for growth cone steering
for example:
attractive or repulsive turning reponses of growth cones to netrin-1 and Semaphorin3A (respectively)
require local translation witin the growth cone
among transcipts regulated by local tranlsation in respoponce to guidance in response to guidance cues are:
beta-actin (attraction)
cofilin
RhoA→ repulsion→ including actin depolymerisation
in summary→ different mRNA activated
difference receptors causes different pools of mRNAs to be activated
how does mRNAs
stya, get there and get pooled
mRNA increase the seniticity of the direction due to the cue sensed by the receptors seen above
What else is also important in growth cone guidance and adaptation and re-sentiisation
local endocytosis of receptors and targeting of components
Summary of growth cone guidance
