Maternal Adaptations to Pregnancy
1/59
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
60 Terms
How much progesterone is being made in late pregnancy and from where?
200mg/day, at first the CL but then maintained by the placenta. Maternal plasma levels are much higher than in the normal luteal phase.
Role of progesterone in pregnancy
supports uterine quiescence
What does the fetus do to progesterone if it gets across the barrier?
The foetus can conjugate steroids to sulfates at the placental barrier, making them biologically inactive.
List pregnancy hormones. (7)
1. Placental prolactin
2. Placental lactogens
3. CRH
4. Aldosterone (plasma vol)
5. EPO (RBC)
6. Cytokines (pro-inflammatory interleukins, TGF-β
7. Vasodilatory mediators - VEGF, NO (angiogenesis, vasodilatation)
What are the roles of placental prolactin?
For breast changes and behavioural changes.
What are the roles of the placental lactogens?
Maternal insulin + glucose metabolism, lipolysis and erythropoiesis.
What are the roles of placental CRH?
Increased cortisol secretion in mother, causing a stress response. Can affect nutrient transfer and placental clock (risks - pre-term labour and early parturition signals).
What anatomical changes occur to the uterus during pregnancy?
1. Uterus enlarges (expands and increases in weight by 20x).
2. Uterine musculature is hypertrophied (needed to expel foetus at parturition).
What happens to myometrial contractions throughout gestation?
They are quiescent, and this is maintained by PGs and oxytocin.
How does the diaphragm change in pregnancy?
Progressively displaced cranially (head side) by the gravid uterus - a 4cm elevation.
How does the heart change in pregnancy? (2)
1. Apex of heart moves anterior and to the left (pushed up and rotated forward).
2. Left ventricular hypertrophy in 1st trimester to cope with increased CO.
Ventricular wall muscle mass increases in the first trimester to cope with maternal cardiac output
How do the bones/skeletal system change in pregnancy?
Changes in Ca2+ conc - more intestinal Ca2+ absorption and bone loss which can occur in 3rd trimester and lactation, but is reversible.
How does the endometrium and mammary gland change in pregnancy?
Decidual changes occur in endometrium to accomodate growing baby.
Mammary gland is developed to form lactating breast.
Why does the mother gain weight during pregnancy?
Increased maternal blood vol and weight of placenta and baby.
How does the blood volume change throughout pregnancy?
- Blood volume increases by 40%
- Red cell mass increases linearly by 30%.
- Plasma vol increases more than cell mass, causing a fall in HCT and Hb.
- But at term, Hb values are still 50% higher than non-pregnant.
What is the advantage of a fall in HCT and Hb?
This is known as physiological anaemia, characterised by less viscosity, lower flow resistance, and improved placental perfusion.
How is plasma volume and red cell mass increased in pregnancy?
Plasma vol: Stimulation of RAAS.
Red cell mass: Increased renal EPO production.
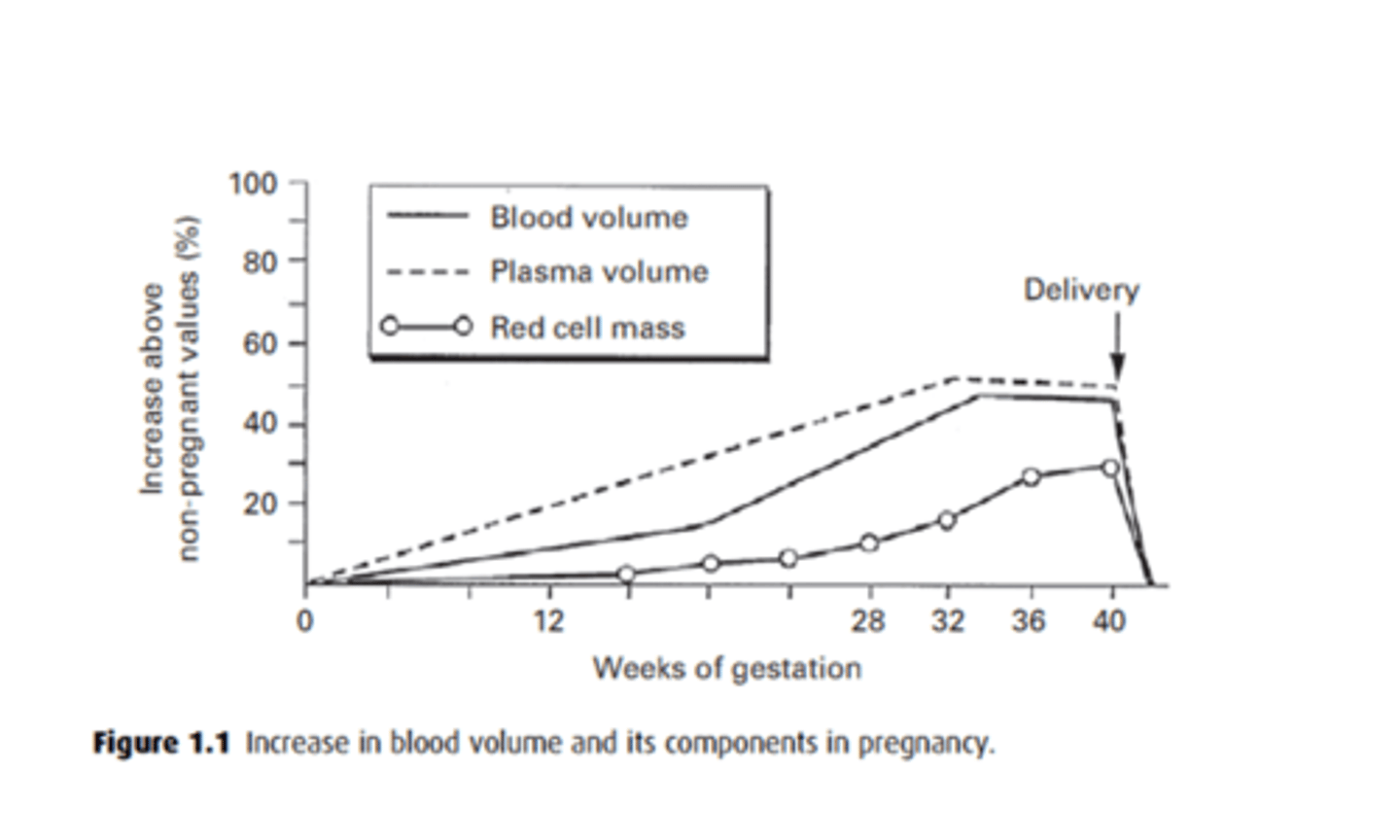
What is the physiological anaemia of pregnancy? How can this be treated?
Renal EPO increases red cell mass by 20-30%, a small rise in comparison to plasma vol which causes haemodilution and decrease in Hb conc from 15 to 12 g/dl.
This is treated with supplemental iron and folic acid intake.
Why is an increase in blood volume a beneficial thing during delivery?
Reduces impact of maternal blood loss at delivery. Autotransfusions of blood from contracting uterus compensates for losses of vaginal births and C-sections.
What causes the reduction of total peripheral resistance in pregnancy? (4)
1. Vascular dilatation
2. Relaxation of peripheral vascular tone
3. Establishing new vascular beds i.e. utero-placental circulation
4. Lowers BP, increasing blood volume.
There is a reduction of 40% by mid-pregnancy, then rising slowly to term.
Describe the hormonal changes that occur in pregnancy
Stimulation of renin-angiotensin system = increased Aldosterone →
acts on the kidney → Retention of sodium and water → increased plasma volume. → Increased renal erythropoietin - increased red cell mass
normally activation of RAAS , causes high angiotensin II . This would Cause vasoconstriction and Increase blood pressure.
But in pregnacy this doesnt occur , why ?
pregnant women are resistant to ang II due to high progesterone (relaxes SM ) , NO + PGI2 (vasodilator)
→ no hypertension
Describe the vascular changes that can been seen in pregnancy
Reduced peripheral resistance (40% decrease by mid-pregnancy).
Formation of new vascular beds (e.g., utero-placental circulation).
Systolic BP stable; diastolic BP drops initially, rises to normal near term.
Why does cardiac output increase during pregnancy
due to higher stroke volume and heart rate
uterine blood flow makes up to 25% of maternal cardiac output -late pregnancy
Which vasoactive factors lead to a reduction in peripheral vascular resistance? (4)
1. VEGF
2. PLGF
3. NO
4. Progesterone
All relax SM of aa.
How does cardiac output change with pregnancy? Why does this happen? Explain the process. (5)
1. Higher blood vol > more blood enters heart (preload)
2. Lower peripheral resistance (vasodilatation) > lower afterload (Pa at which ventricle ejects blood)
3. Leads to higher stroke vol (amount of blood ejected from ventricle or into aorta per cycle)
4. Increase in maternal HR
5. Thus increase in CO (SV X HR).
This extra CO is because extra 30-50mL of O2 is consumed per min during pregnancy.
How much CO does blood flow to uterus and placenta constitute? Does this fall towards term?
Up to 25% of maternal CO. This does not fall towards term.
How does CO change with established labour?
Pushes CO up by another 2L/min.
How does systolic BP change throughout pregnancy?
Remains stable. In early pregnancy, diastolic Pa falls, but rises to normal values by term.
What effect does the enlarging uterus in pregnancy have on the aorta and inferior vena cava? What are the consequences?
Compresses both when patient is supine.
IVC compression: Reduces venous return to heart (drop in preload and CO). Can be severe and lead to loss of consciousness.
Aortic compression: Reduced uteroplacental and renal blood flow.
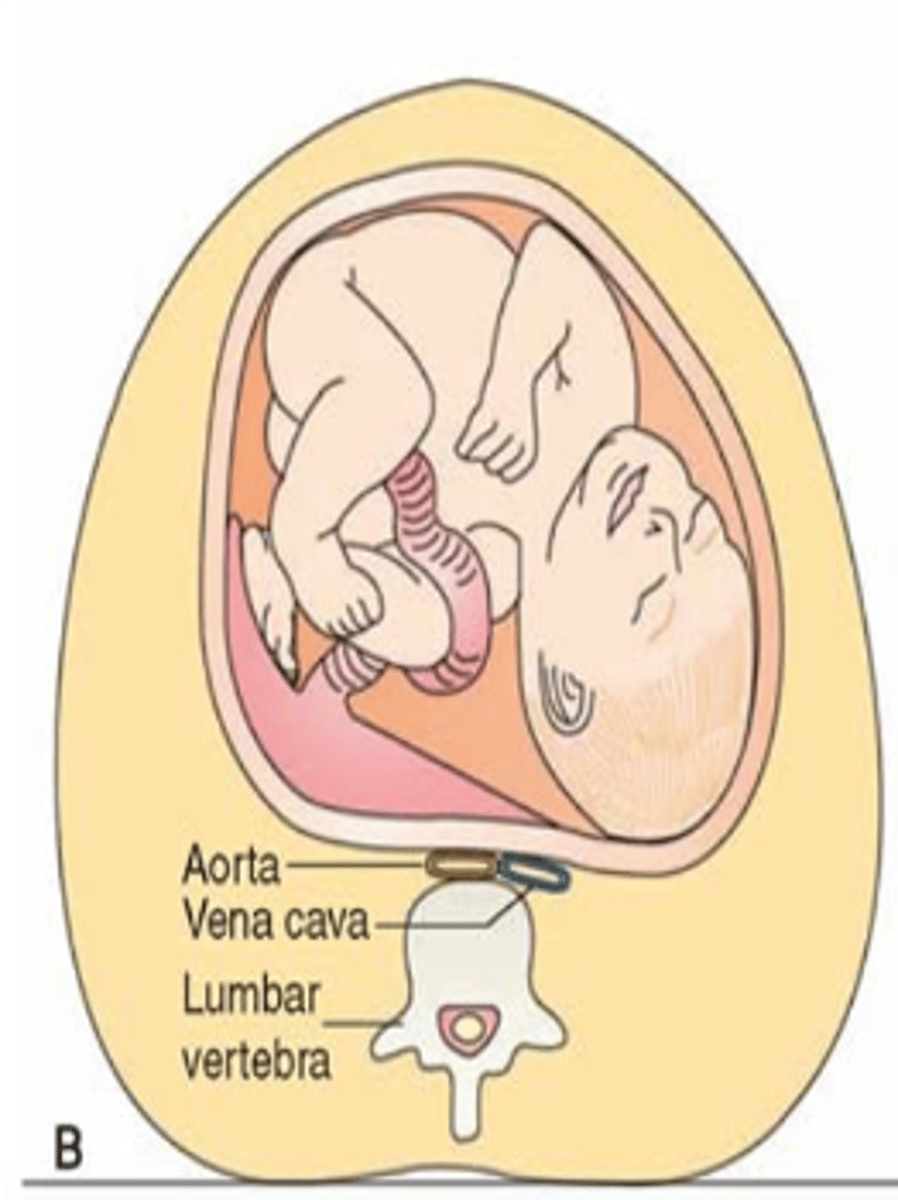
What is the maternal kidney function like during the last trimester? What is the ruling around women lying down in late pregnancy?
Markedly lower when supine than in lateral position. No woman should lie supine in late pregnancy.
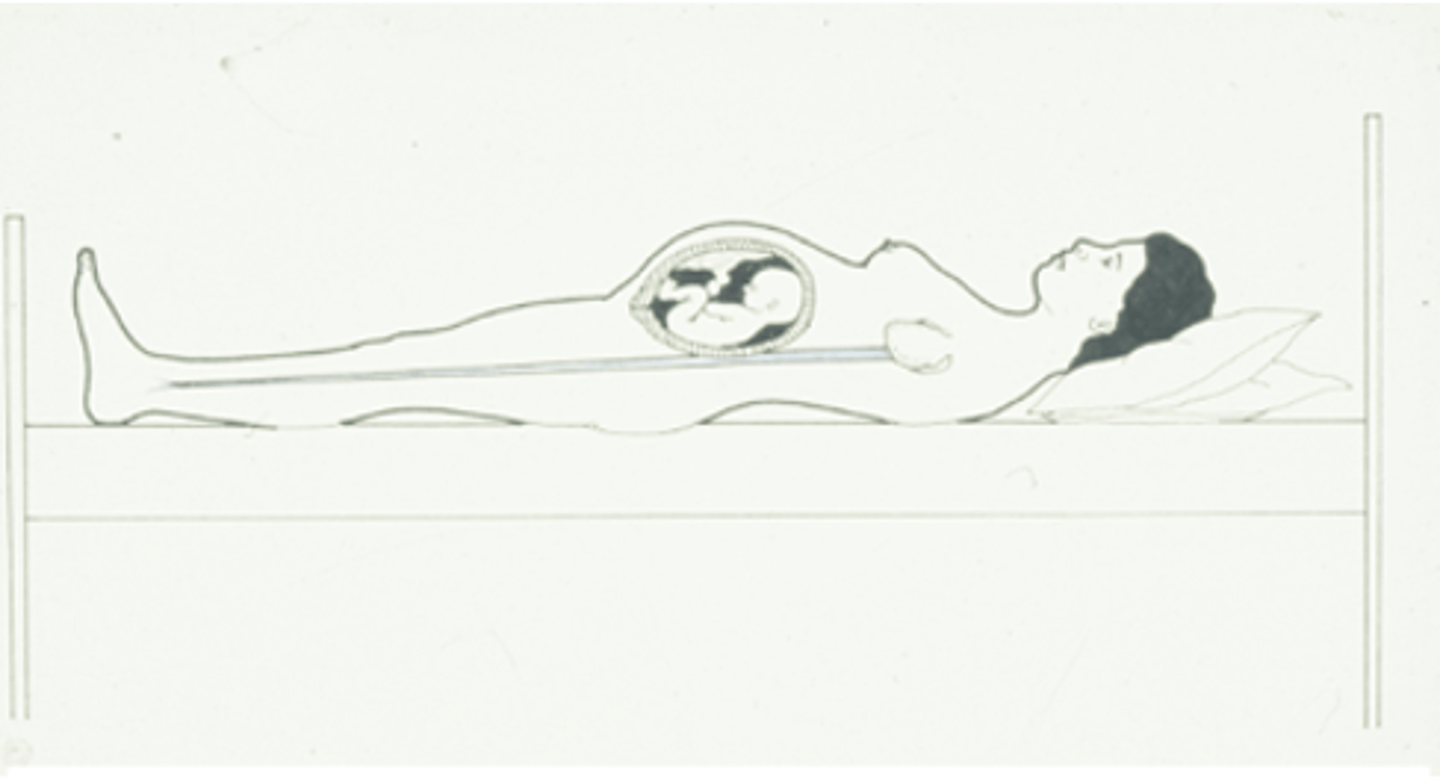
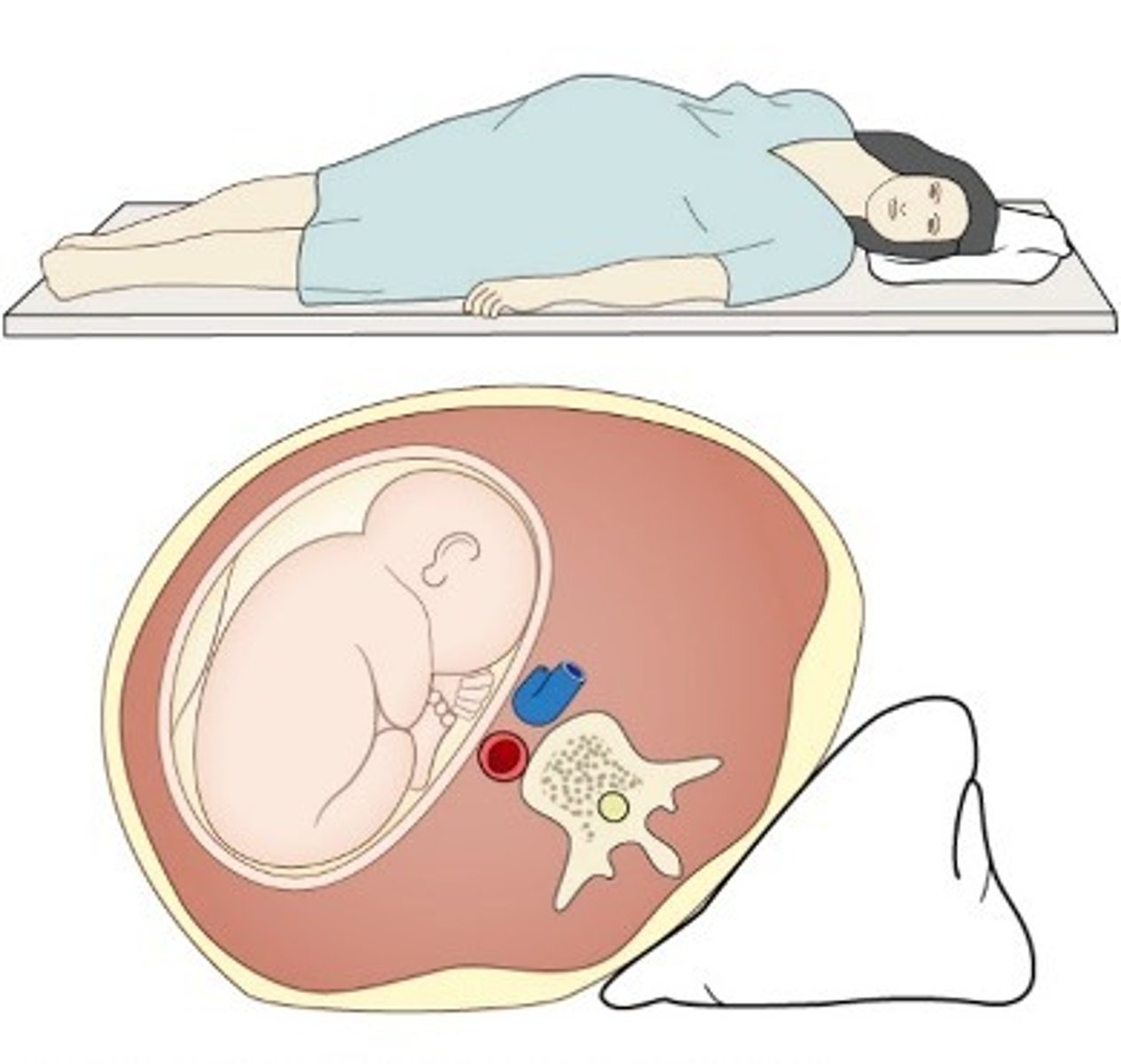
Why should women not lie supine in late pregnancy
since the aorta and IVC are already compressed
reduced preload → restrict blood flow to the below > effects gas exchange → so should lie on the left
What position are mothers placed during labour?
On a left lateral tilt.
What are the structural changes that occur in the respiratory system as a result of pregnancy? How does this affect O2 consumption? (3)
O2 consumption increases in all tissues.
1. Increased chest expansion
2. Displaced diaphragm
3. Increased vascularisation of upper RT.
What 3 things does the progesterone-mediated hypersensitivity to CO2 lead to in pregnancy?
1. increases VR by 15%
2. increases TV by 40%
3. increases AVR by 70% by the end of gestation
What are the consequences of pCO2 and alveolar pCO2 decreasing, and pO2 increasing in pregnancy? (2)
Higher PaO2 on maternal side of placenta facilitates oxygen transfer to fetus
Lower PaCO2 on maternal side of placenta facilitates CO2 transfer from fetus
Can restore by keeping alveolar pressure high
What are the anatomical changes occurring to the kidney during pregnancy? (4)
1. Enlarged kidneys (1cm) due to more vasculature, vascular dilatation and increase in interstitial space.
2. Increased renal parenchyma volume, higher diameter of Bowman's capsule.
3. Dilatation of calyces, renal pelvis and ureter (due to progesterone and local Pa effect). Increases chances of UTI.
4. Bladder loses tone - high urinary frequency and urgency.
What are the physiological changes occurring to the kidney during pregnancy? (3)
1. Increase in RPF, decrease in renal vascular resistance
2. Changes in GFR (rises by 20-40%) and FF
3. Changes in tubular reabsorption.
More sodium = retain water
Glosyuria = increased glucose filtration/ excretion → risk of UTI
How does filtration fraction change during pregnancy and why?
It decreases. (eraly pregnency)FF = GFR/RPF.
RPF increases by 50-80%, whilst GFR also increases but not that much.
but then rises during mid /late pregnacy → gets to the same level as non pregnent
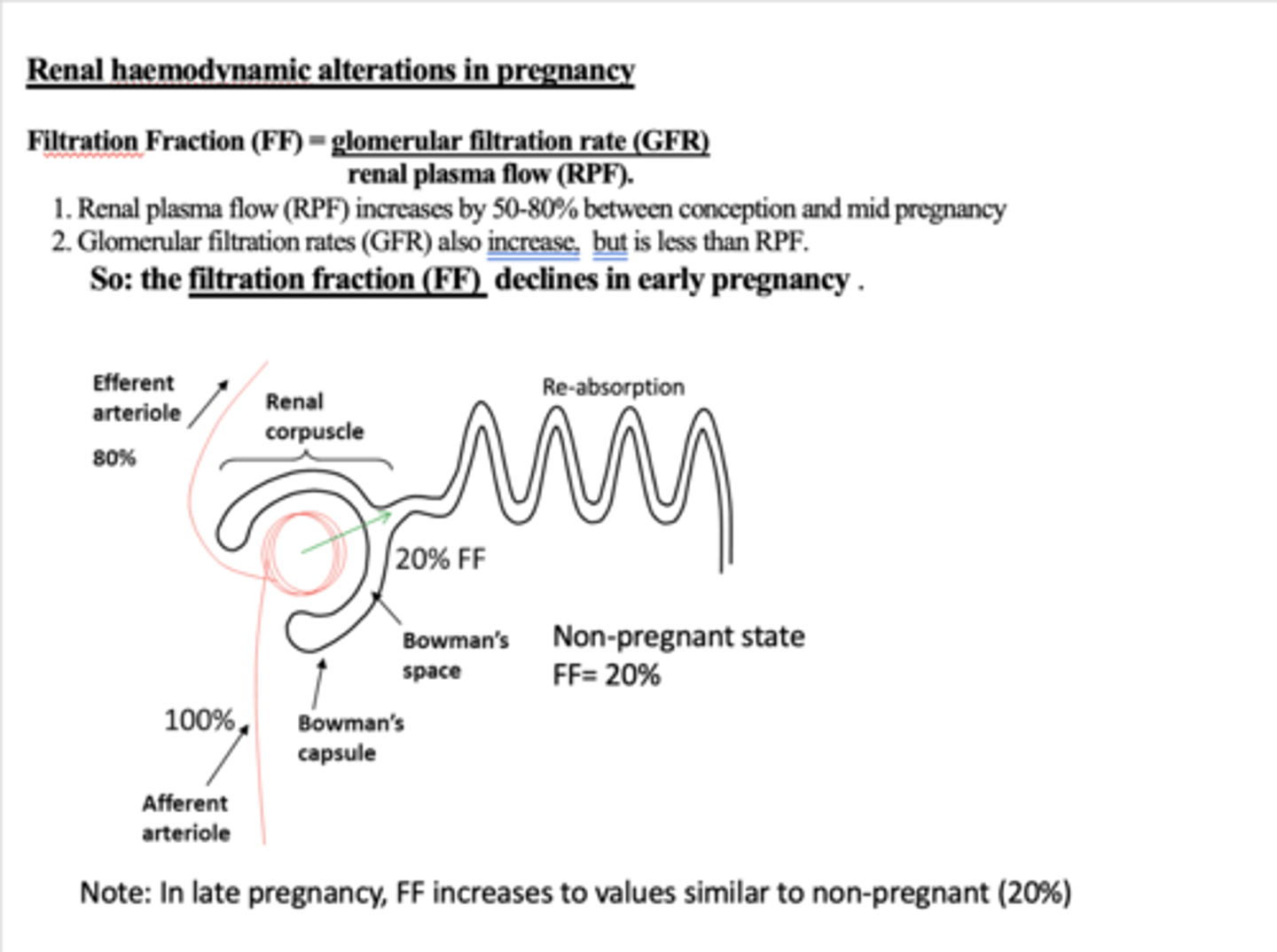
Why does glycosuria occur in pregnant women?
FF declines, but renal flow increases so there is more glucose in filtrate and exceeds max. absorption rates. Means urine contains glucose and can be 10x higher than outside pregnancy.
Increases chances of UTI.
How does the foetus obtain glucose?
Has little capacity for gluconeogenesis, so gets all of it from mother which readily crosses placenta. The maternal glucose levels determine fetal levels.
What is the evidence for glucose deficit happening in mothers? (3)
1. Fasting BG is 10mg/dL lower than non-pregnant
2. Ketones seen in maternal blood
3. Lipolysis and ketosis occur during starvation, therefore pregnancy can be said to be accelerated starvation
Explain the metabolic changes that happen in early pregnancy.
Maternal glucose determines fetal glucose level
Fasting blood glucose value is 10mg
Progesterone boosts appetite and glucose stored in fat stores (promoting lipogenesis)
Increased insulin sensitivity in early pregnancy, which promotes fat storage and glucose uptake
Foetal glucose is dependent on maternal supply.
Explain the metabolic changes that occur in mid-pregnancy?
Increased glucose absorption, gluconeogenesis, total glucose production,and lipolysis.
Progressive insulin resistance; glucose levels stabilize.
In skeletal muscle
plasam glucose returns back to normal after 20 weeks
What is the evidence against glucose deficit happening in mothers?
Although fasting hypoglycaemia occurs in 1st trimester, the drop in glucose levels reach lowest at 12w gestation. This returns to normal in 2nd and 3rd trimesters.
What happens to the insulin sensitivity of the maternal tissue throughout pregnancy?
It becomes more insensitive to insulin (50-80% drop in SkM) so mother takes up less glucose, sparing for foetus instead.
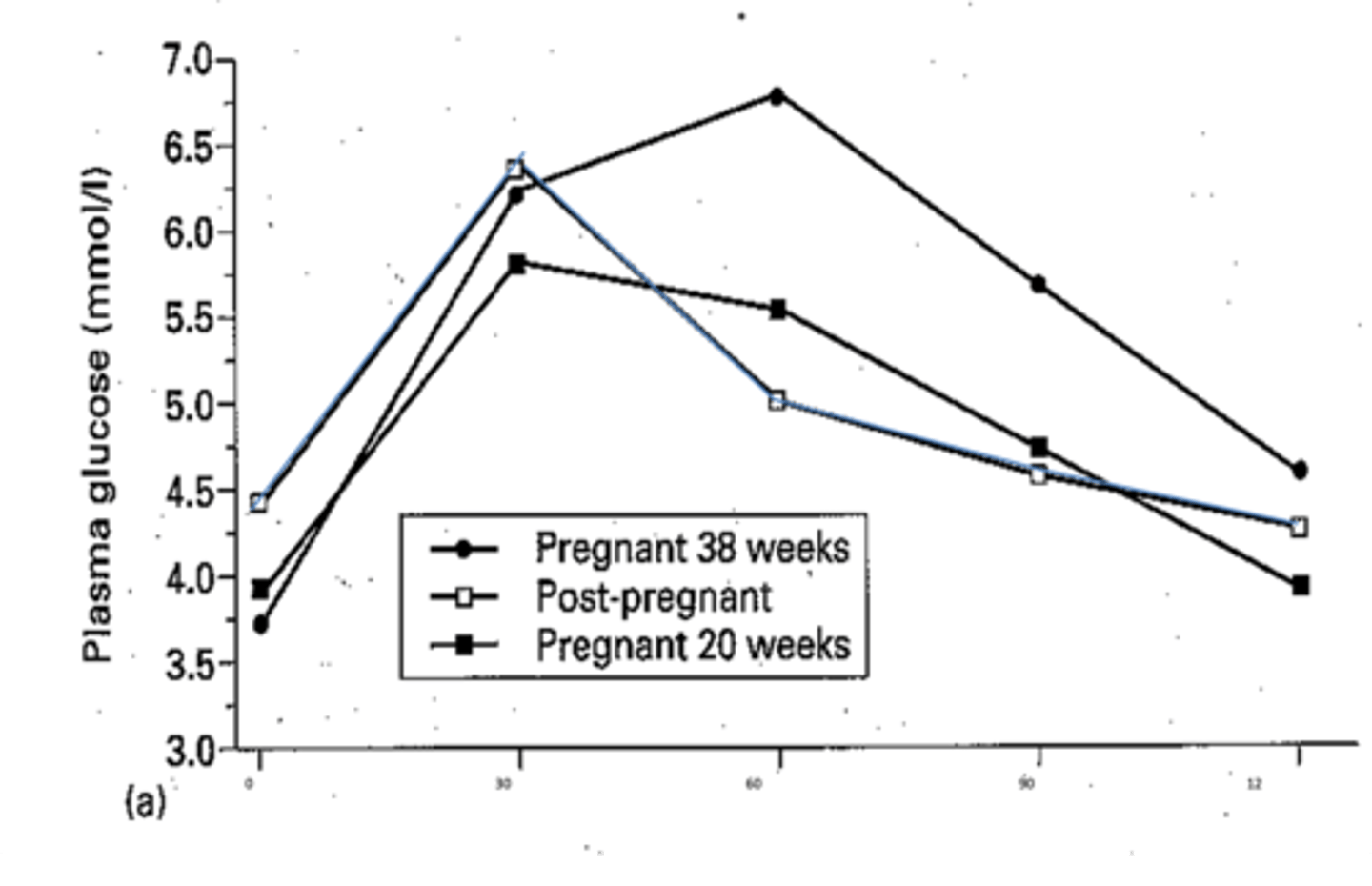
How do the glucose levels change in response to glucose load during pregnancy?
There is a prolonged duration of post-prandial hyperglycaemia in pregnancy.
In the 3rd trimester (38w, solid line dots in graph) plasma glucose takes longer to reach peak, which is a higher peak. Returns to baseline slowly after 2h.
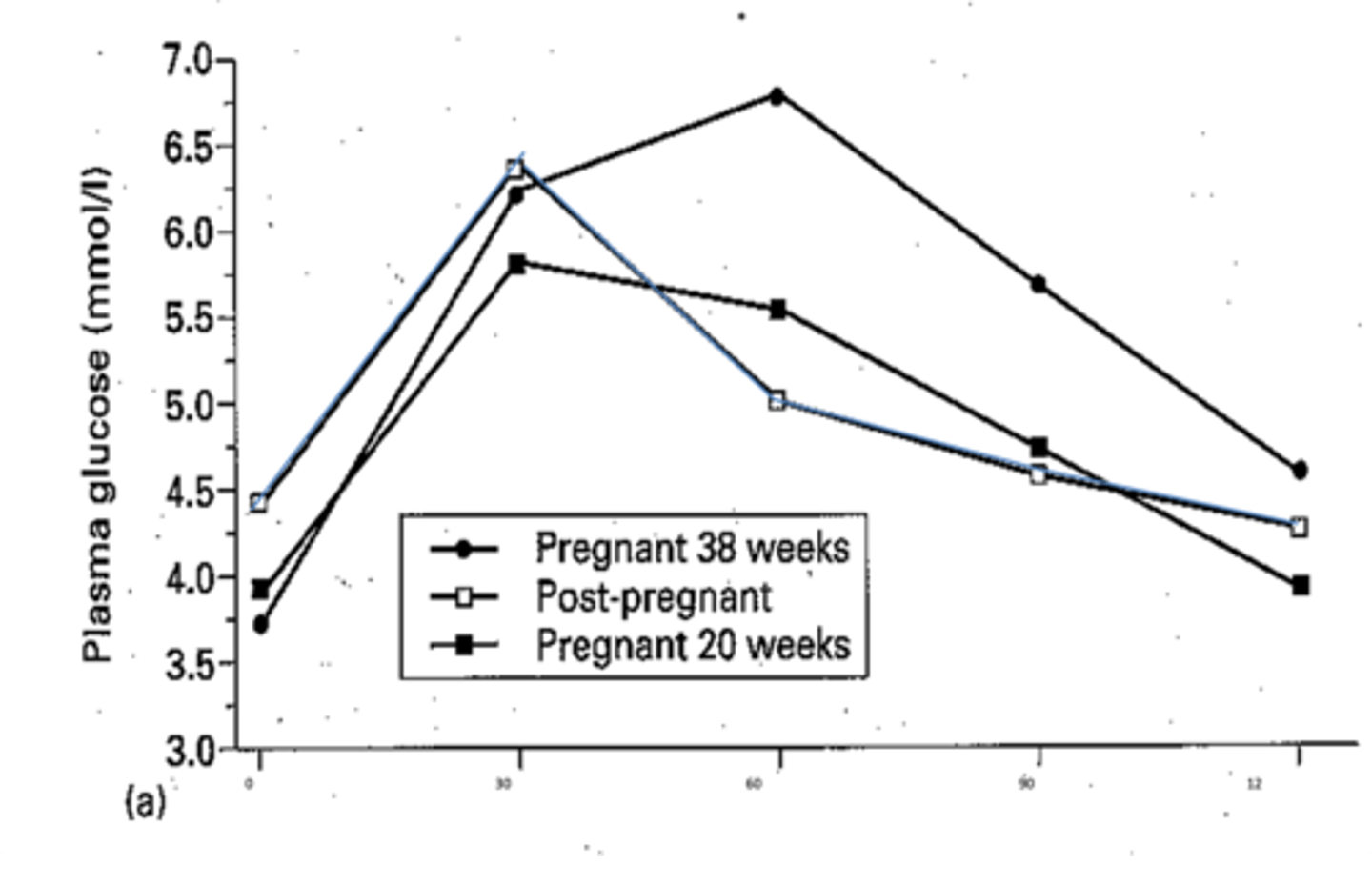
How do the glucose levels change in response to glucose load post-pregnancy?
Blue line in diagram.
Plasma glucose reaches peak 30m after ingestion and returns to baseline after 60m.
How does insulin respond to glucose load during pregnancy?
There is post-prandial hyperinsulinaemia, but also insulin resistance.
In 3rd trimester there is a higher glucose peak so more insulin is secreted. Insulin reaches peak after 1h, which then slowly declines but does not return to basal levels in pregnancy.
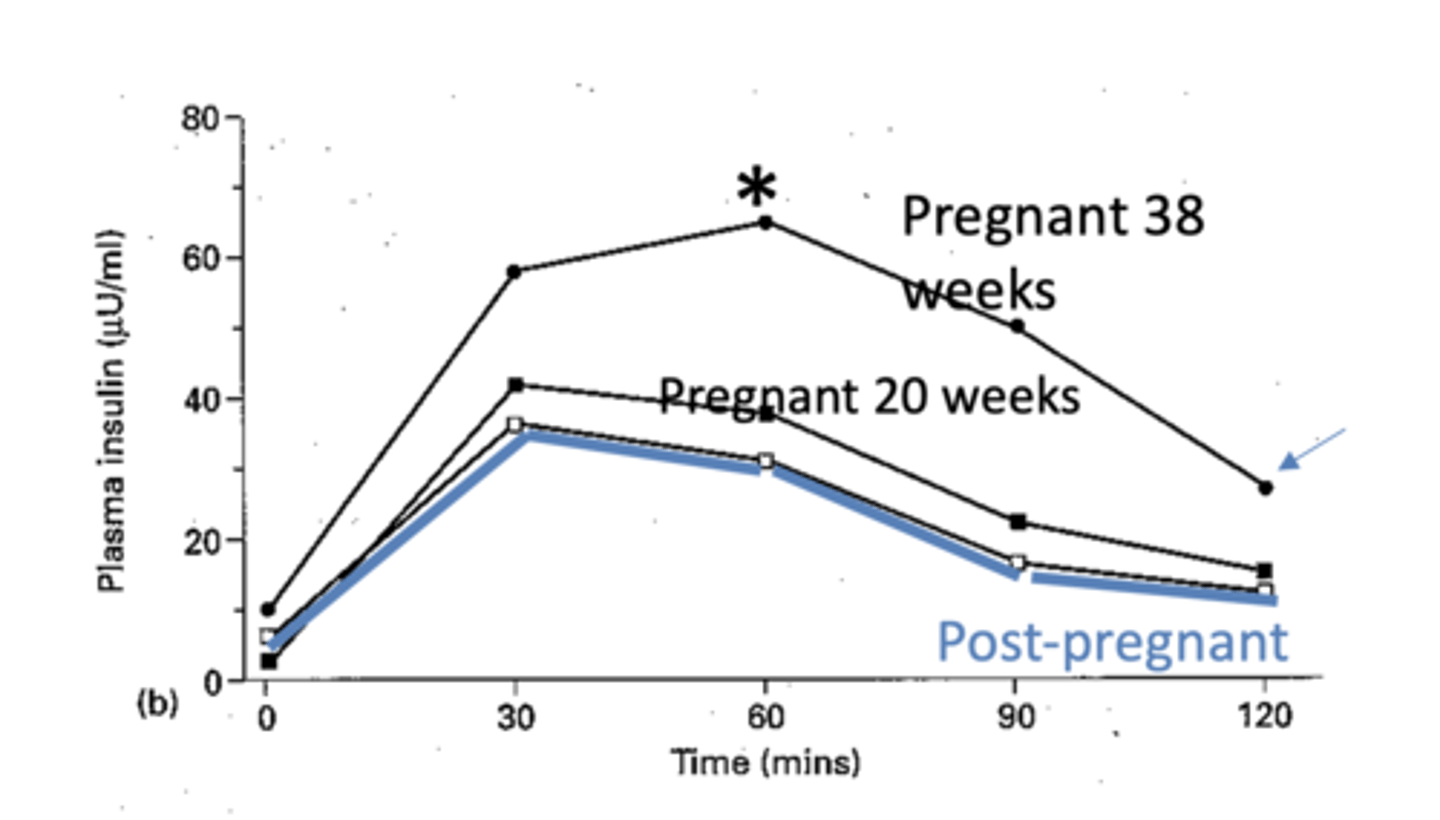
In which pregnancy-related conditions is insulin resistance markedly higher?
Gestational diabetes and maternal obesity.
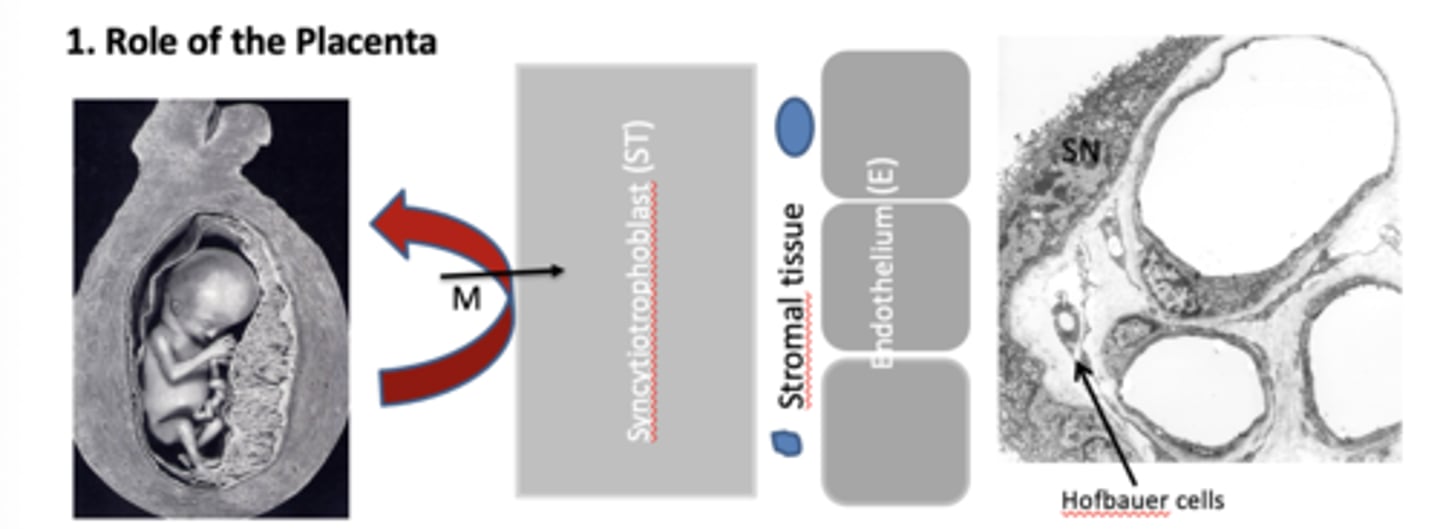
How does the syncytiotrophoblast contribute to the fetus avoiding maternal rejection? (4)
1. Placenta has no histocompatibility antigens (HMCs).
2. Foetus hides behind placenta physically
3. Maternal immune cells can't cross to foetus without going through the cytoplasm
4. If transcytosis does occur, Hofbauer cells will phagocytose maternal immune cells.
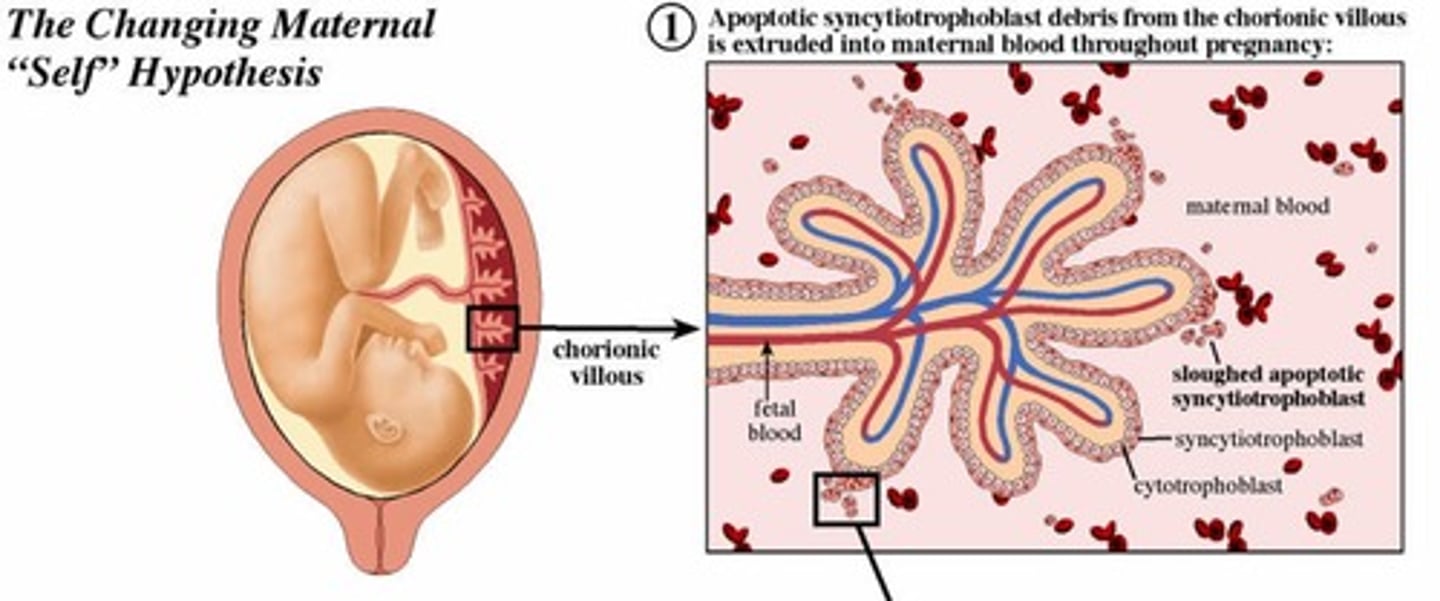
What is the changing maternal 'self' hypothesis? (5)
This is so the mums body DOES NOT see the baby as foreign
Syncytial knots, exosomes, cell-free DNA and fetal stem cells (all these are released by the baby) are phagocytosed by the maternal immune system (phagocytosed)
2. The phagocytosed debris contains intracellular fetal HLA (class I and II) {like the babys ID}
3. Maternal dendritic cells present the fetal peptides to T cells in endometrium.
4. Induces T cell apoptosis and conversion to T reg suppressor cells.{mums body recognises baby as not foreign}
5. Peripheral tolerance of fetal HLA develops.
What are the immune cells that are present in the endometrium? (4)
1. Dendritic (antigen-presenting) cells
2. Th cells
3. Treg cells
4. Uterine NK cells (resident lymphocytes)
How do pregnancy hormones influence the immune cells of the endometrium?
Th cells (which activate immune cells) decline in comparison to the suppressor cells or Treg cells in endometrium. These cells decrease immune function by suppressing responses from the dwindling T-cells and maintain materno-fetal tolerance.
How are the uterine NK cells suppressed in pregnancy, and why does this happen?
Decidual stromal cells secrete endometrial glycoproteins which suppress uNK cells. This happens because uNKs can make cytokines that kill foetal cells. Help maintain materno-fetal tolerance.
What are placental galectins?
Secreted by placenta, have immunosuppressive function and help maintain materno-fetal tolerance.
What is the role of the anti-inflammatory factors (TGF-β and IL-10), and what are they produced by in pregnancy?
Produced by decidua and placenta. Aid apoptosis of T-cells and their conversion to Treg.
What is the role of EVTs in the production of materno-fetal tolerance?
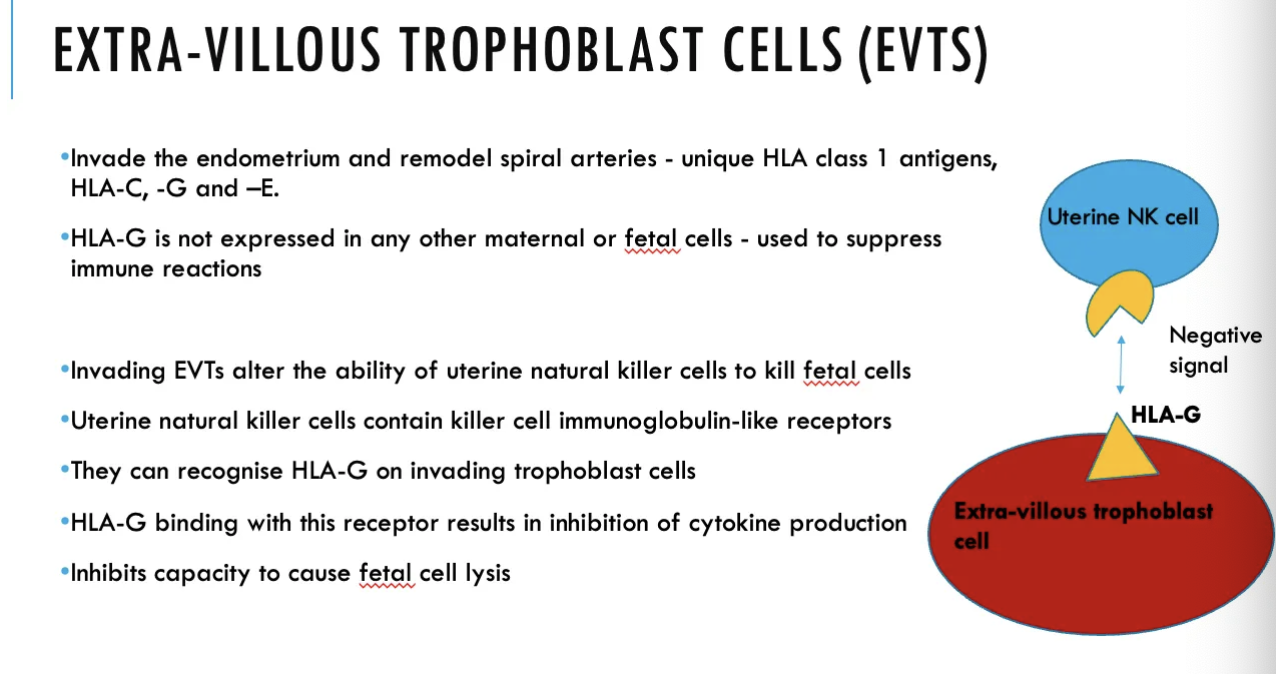
They have a HLA-G antigen, which uNK cells can recognise and bind to using KIR on their surfaces. Leads to inhibition of cytokine production from uNK cells (a -ve signal), so uNKs can't cause foetal cell lysis (and kill foetus), controlling maternal immune response
Generally, what happens to the transfer of maternal Ig to foetus?
Any present antibodies that are against foetal antigens are mostly diluted out by circulating antigens in the foetus.
How does Rhesus disease occur?
Usually only affects the next pregnancy.
If mother is Rh-ve but her current baby is Rh+ve, she can develop an immune response against the +ve antigens if her previous child was also Rh+ve. In the first pregnancy, she would have developed antigens against the +ve antigen, which means in the subsequent pregnancy, maternal IgG cross placenta and cause fetal erythroblast destruction in Rh+ve foetus.