Atomic Models
1/5
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
6 Terms
Dalton's Model
States that atoms are like a smooth, solid sphere that cannot be broken down into smaller pieces.
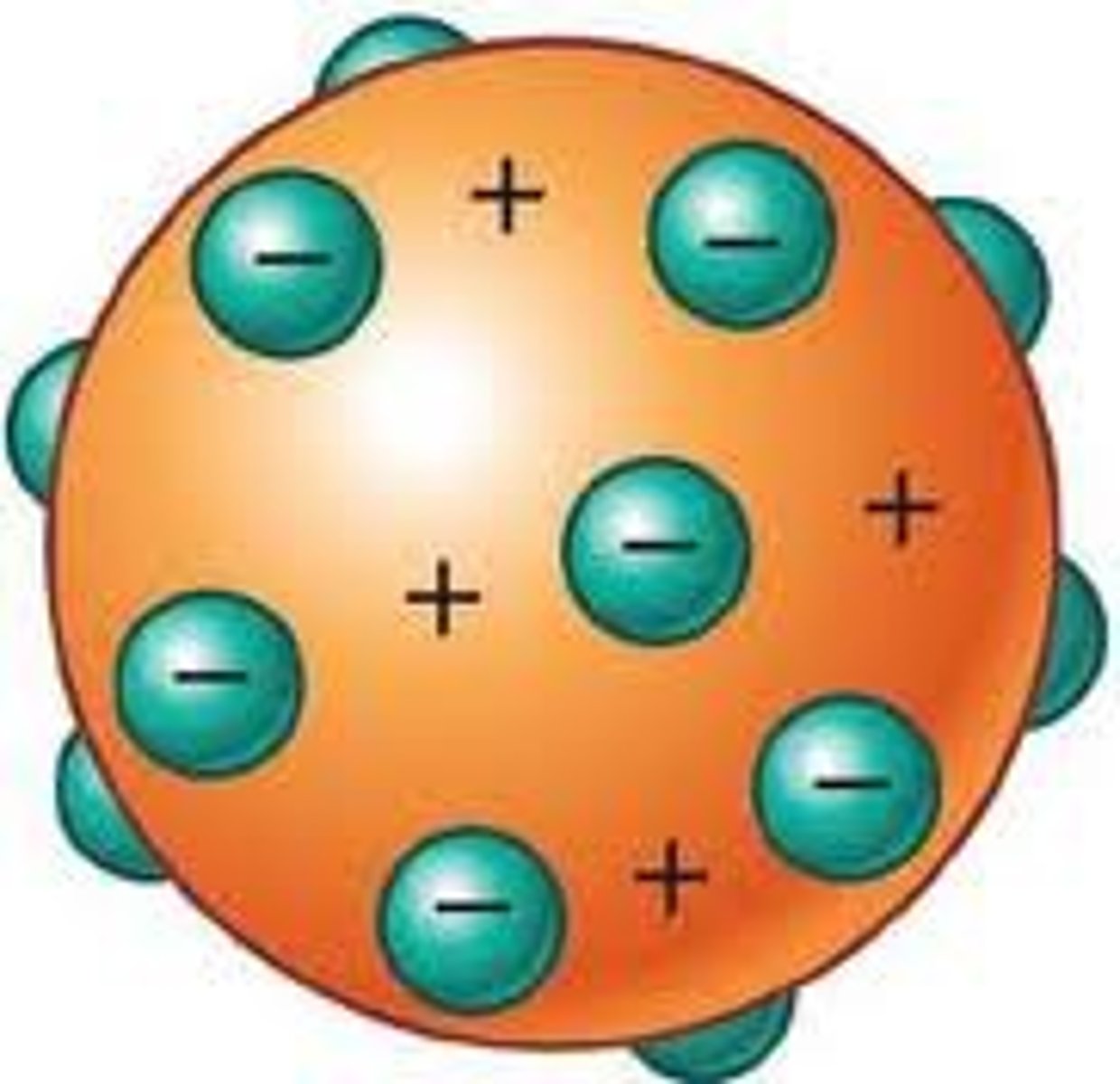
Thomson's Model
States that atoms are negatively-charged particles are imbedded in positively-charged sphere, like chocolate chips in a cookie.
Rutherford's Model
Atoms are mostly empty space filled with electrons. He proved atoms have a positively-charged nucleus when a stream of positive particles shot through gold foil were deflected; two positive particles repel one another. He named these positive particles in the nucleus "protons."
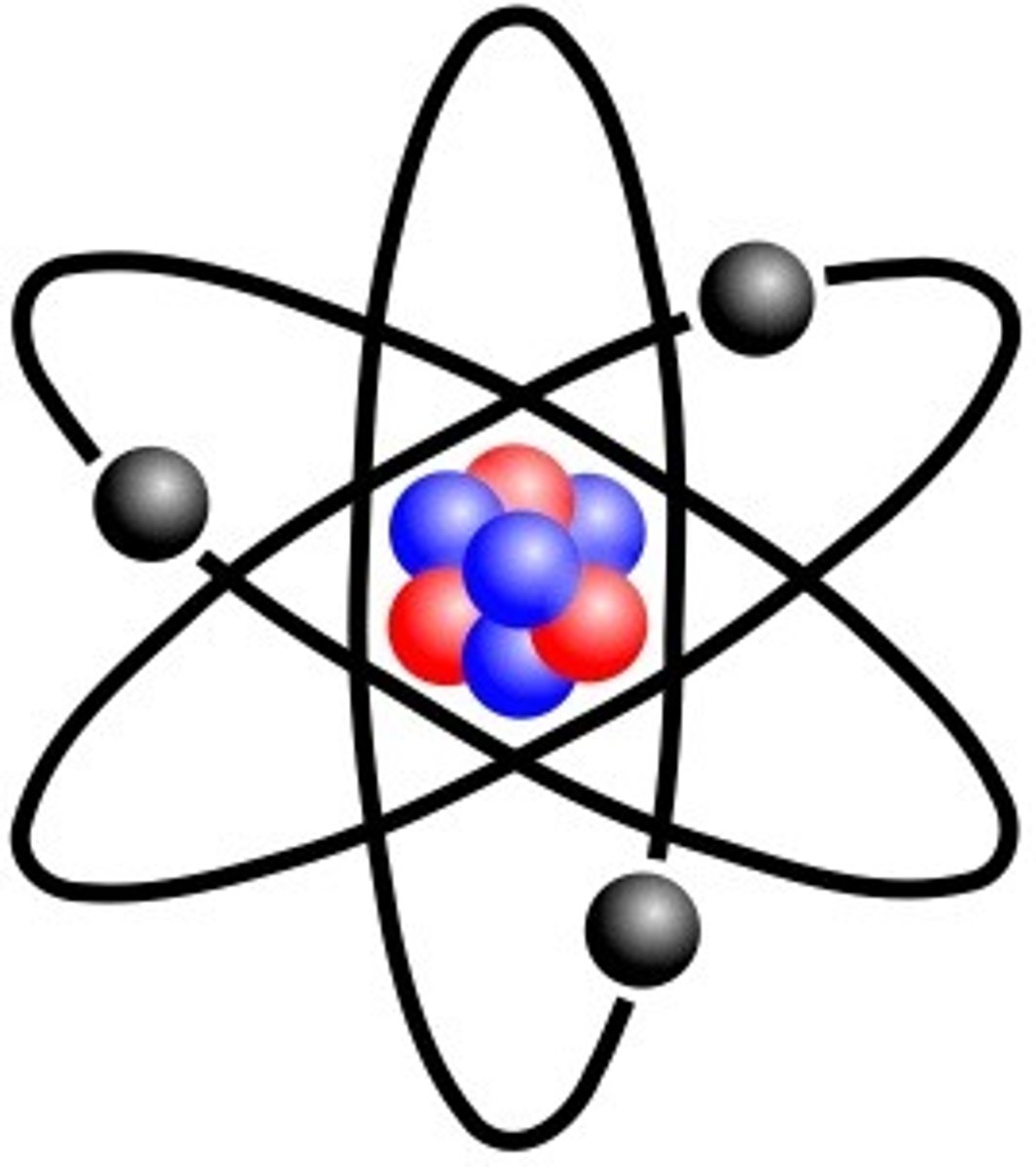
Bohr's Model
His model resembled a cut onion, like rings. He believed electrons orbited around the nucleus like planets around the sun.
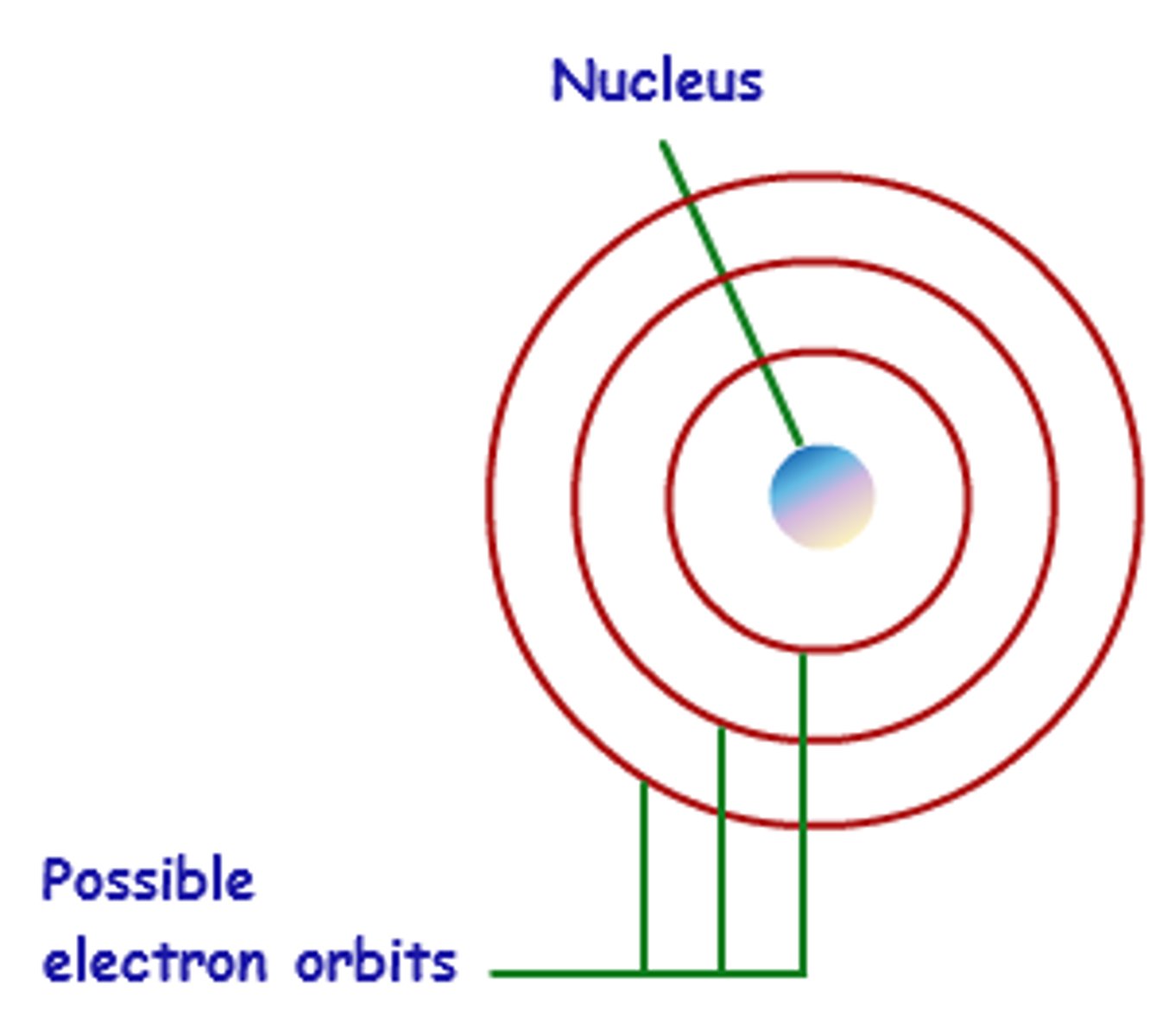
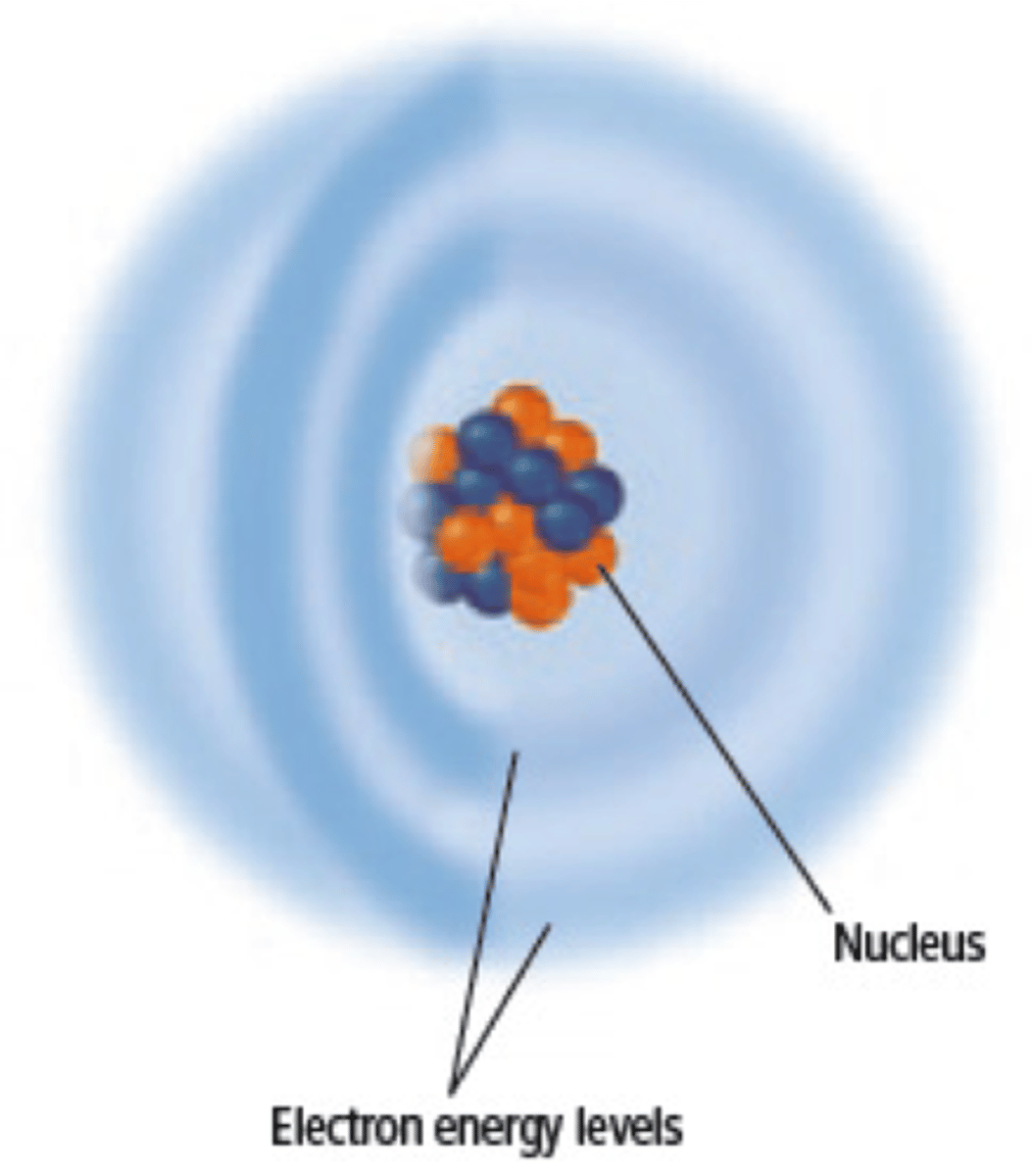
Cloud Model
Unlike Bohr's model, the Cloud model states the electrons are anywhere around the nucleus in a cloud of specific energy levels.
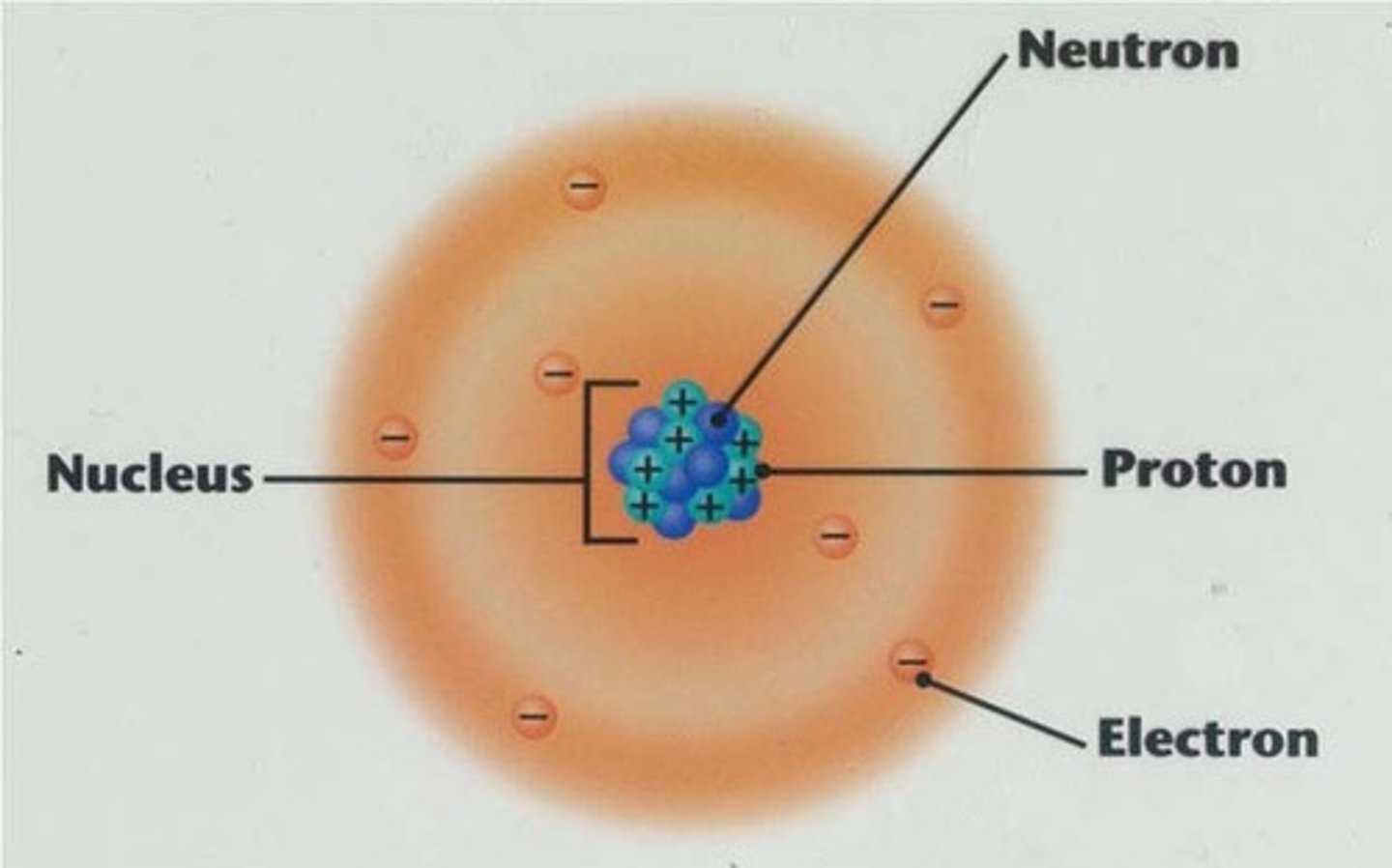
Modern Model
This model, developed in the 1930s and still used today, describes the atom with protons and neutrons making up an atom's nucleus and electrons in a cloud surrounding it.