properties of organic molecules (new material)
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
13 Terms
primary carbon
1o
carbon that is bonded to only one other carbon
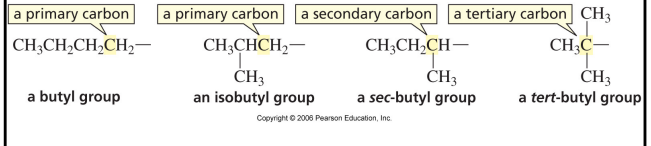
secondary carbon
2o
carbon that is bonded to two other carbons
tertiary carbon
3o
carbon bonded to three other carbons
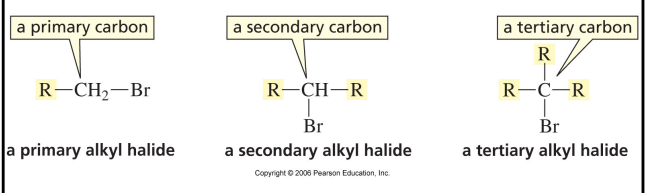
classification of alkyl halides
number of alkyl groups attached to carbon with halogen bonded to it determines whether alkyl halide is primary, secondary or tertiary
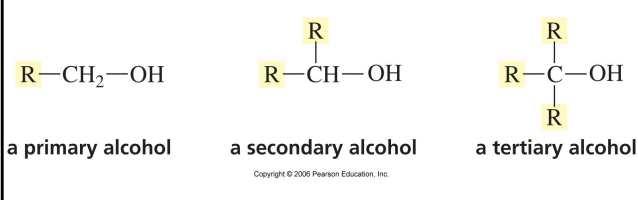
classification
number of alkyl groups attached to carbon to which OH group is attached determines whether alcohol is primary, secondary or tertiary
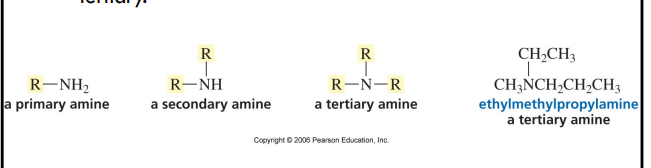
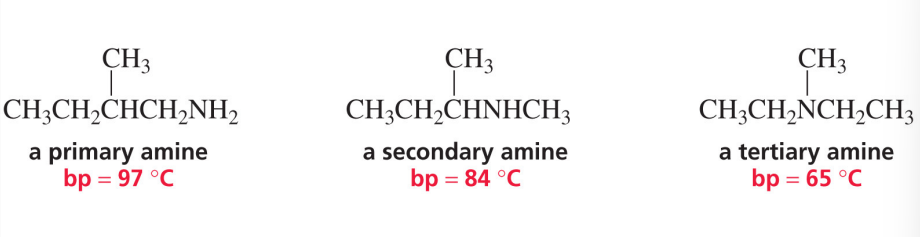
classification of amines
number of alkyl groups attached to the nitrogen determines whether an amine is primary, secondary, or tertiary
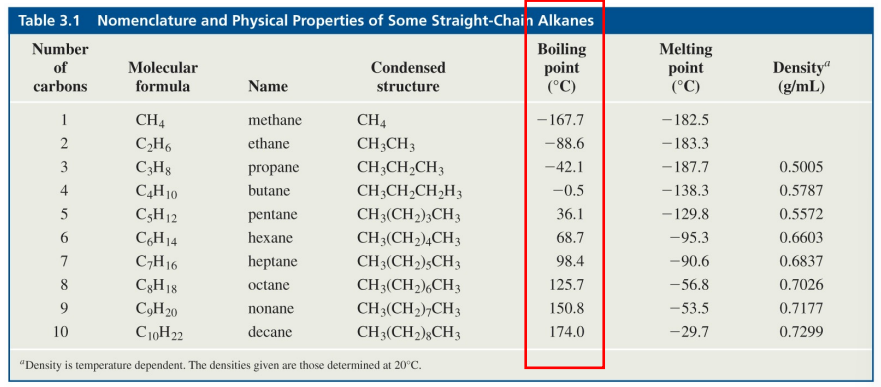
boiling of points of alkanes
boiling points increase with increasing molecular weight within a homologous series of alkanes
branches decrease area of contract between molecules, so more branches = lower boiling point
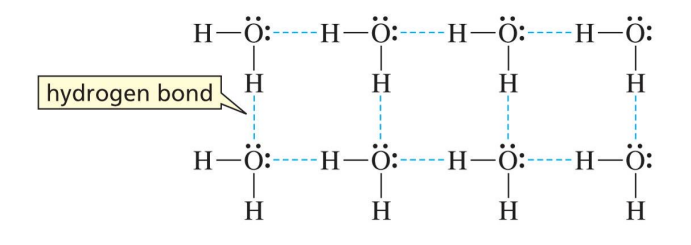
boiling point and alcohol
have higher boiling points than alkanes or ethers or comparable molecular weight
need to break london dispersion, dipole dipole and hydrogen bonds
amines and boiling point
primary and secondary amines also form hydrogen bonds
these will have higher boiling points than alkanes with similar molecular weights
melting points of alkanes
temperature at which a solid is converted into a liquid
increase in mp is not as regular as increase in bp because of packing
packing: property that determines how well the individual molecules in a solid fit together in a crystal lattice
melting point increase with increasing molecular weight within a homologous series of alkanes
solubility
like dissolves like
polar solutes dissolve in polar solvents
nonpolar solutes dissolve in nonpolar solvents
molecules with similar intermolecular forces will mix freely
water is the universal solvent
organic compound solubility
most organic molecules are usually soluble in organic solvents (organic molecule must contain C and H)
some organic molecules soluble in water → indicates high ratio of polar groups to carbon chain
size and symmetry of function groups
add after lesson