BCMB 3100 Test 2
0.0(0)
Card Sorting
1/44
Earn XP
Description and Tags
Last updated 4:41 PM on 10/12/22
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
45 Terms
1
New cards
Vmax
def: maximum velocity when all enzymes are saturated with substrate
dependent on enzyme concentration
dependent on enzyme concentration
2
New cards
kcat (K2)
turnover number which is achieved at Vmax
rate constant of rate-limiting step of the reaction
Kcat = Vmax/ [E]total (at high [S], [E] total would be all in ES complexes so really its Vmax/ ES concentration)
rate constant of rate-limiting step of the reaction
Kcat = Vmax/ [E]total (at high [S], [E] total would be all in ES complexes so really its Vmax/ ES concentration)
3
New cards
Km
stability of enzyme-substrate complex
compilation of rate constants: Km = (K-1+K2)/K1
(ES falls apart/ES forms)
independent of enzyme concentration
compilation of rate constants: Km = (K-1+K2)/K1
(ES falls apart/ES forms)
independent of enzyme concentration
4
New cards
catalytic efficiency
=Kcat/Km
how fast the ES makes product
---------------------------------------------------
how easily ES is formed
larger = more efficient enzyme
how fast the ES makes product
---------------------------------------------------
how easily ES is formed
larger = more efficient enzyme
5
New cards
Michaelis-Menten Plot (how to find Vmax and Km)
Vmax is the asymptotic value that the MM plot attempts to reach at high [S] values
Km is the [S] at Vmax/2
Km is the [S] at Vmax/2
6
New cards
hallmark behavior of saturated catalyst in MM plot
starts as a first order with respect to [S] (linear phase) and then becomes a zero order at high [S] because rate stops depending on [S]
at high [S] values all enzymes are utilized in ES complexes
at high [S], V0 = K2 x [ES]
at high [S] values all enzymes are utilized in ES complexes
at high [S], V0 = K2 x [ES]
7
New cards
how to calculate Vmax and Km on Lineweaver-Burk plots
1/Vmax= y-int
slope= Km/Vmax
slope= Km/Vmax
8
New cards
irreversible v reversible inhibitors
Irreversible- inhibitor covalently binds to groups on the active sites of an enzyme
reversible - inhibitor noncovalently binds to site on enzyme
reversible - inhibitor noncovalently binds to site on enzyme
9
New cards
3 types of reversible inhibitors
competitive: attaches at active site
- Vmax: unchanged
- Km: increased
-can be overcome by increase [S]
noncompetitive: attaches at allosteric site
-Vmax: reduced
-Km: unchanged
-cannot be overcome by increasing [S]
uncompetitive: attaches at group of the active site
-Vmax: reduced
-Km: reduced
-cannot be overcome by increasing [S]
- Vmax: unchanged
- Km: increased
-can be overcome by increase [S]
noncompetitive: attaches at allosteric site
-Vmax: reduced
-Km: unchanged
-cannot be overcome by increasing [S]
uncompetitive: attaches at group of the active site
-Vmax: reduced
-Km: reduced
-cannot be overcome by increasing [S]
![competitive: attaches at active site
- Vmax: unchanged
- Km: increased
-can be overcome by increase [S]
noncompetitive: attaches at allosteric site
-Vmax: reduced
-Km: unchanged
-cannot be overcome by increasing [S]
uncompetitive: attaches at group of the active site
-Vmax: reduced
-Km: reduced
-cannot be overcome by increasing [S]](https://knowt-user-attachments.s3.amazonaws.com/2afe8670fc384e7f9ab8cef62efc51a3.jpeg)
10
New cards
anabolism vs catabolism
anabolism- creating molecules from smaller components; biosynthesis
- example: gluconeogenesis
catabolism- breakdown of larger molecules into simple components
- example: glycolysis
- example: gluconeogenesis
catabolism- breakdown of larger molecules into simple components
- example: glycolysis
11
New cards
Redox reactions
reactions in which one species loses an electron and another gains it
LEO says GER
LEO says GER
12
New cards
electron carriers in metabolism
NADH and FADH2
help power metabolic pathways - redox reactions ; coupling reactions
help power metabolic pathways - redox reactions ; coupling reactions
13
New cards
Reduced and oxidized forms of NAD and FAD
NAD:
reduced: NADH
oxidized: NAD+
FAD:
reduced: FADH2
oxidized: FAD
reduced: NADH
oxidized: NAD+
FAD:
reduced: FADH2
oxidized: FAD
14
New cards
Comparing single carbon molecules based on free energy of oxidation, stability, energy, and usefulness as fuel
more energy= better as fuel, higher free energy of oxidation, less stable
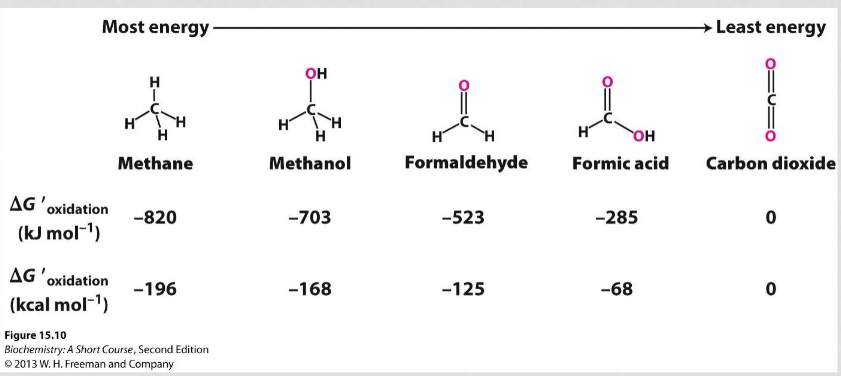
15
New cards
why is ATP the energy currency in biology?
ATP hydrolysis is an energetically favored reaction with a negative delta G
ATP also has a very high phosphoryl transfer potential which means it is very likely to give up a P and release energy in the process
-(due to electrostatic repulsion, stabilization due to hydration, increase in entropy, and resonance stabilization)
ATP also has a very high phosphoryl transfer potential which means it is very likely to give up a P and release energy in the process
-(due to electrostatic repulsion, stabilization due to hydration, increase in entropy, and resonance stabilization)
16
New cards
based on the delta G, which steps are irreversible
steps 1, 3, and 10
17
New cards
identify redox reactions in glycolysis
step 6 -
glyceraldehyde-6-phosphate --> 1,3-bisphosphoglycerate
and
NAD+ +Pi --> NADH + H
glyceraldehyde-6-phosphate --> 1,3-bisphosphoglycerate
and
NAD+ +Pi --> NADH + H
18
New cards
identify a step that shows phosphoryl transfer potential
step 7-
1,3-bisphosphoglycerate --> 3-phosphoglycerate
and
ADP--> ATP
1,3-bpg has a higher phosphoryl transfer potential than ATP so it gave up a P to create ATP
1,3-bisphosphoglycerate --> 3-phosphoglycerate
and
ADP--> ATP
1,3-bpg has a higher phosphoryl transfer potential than ATP so it gave up a P to create ATP
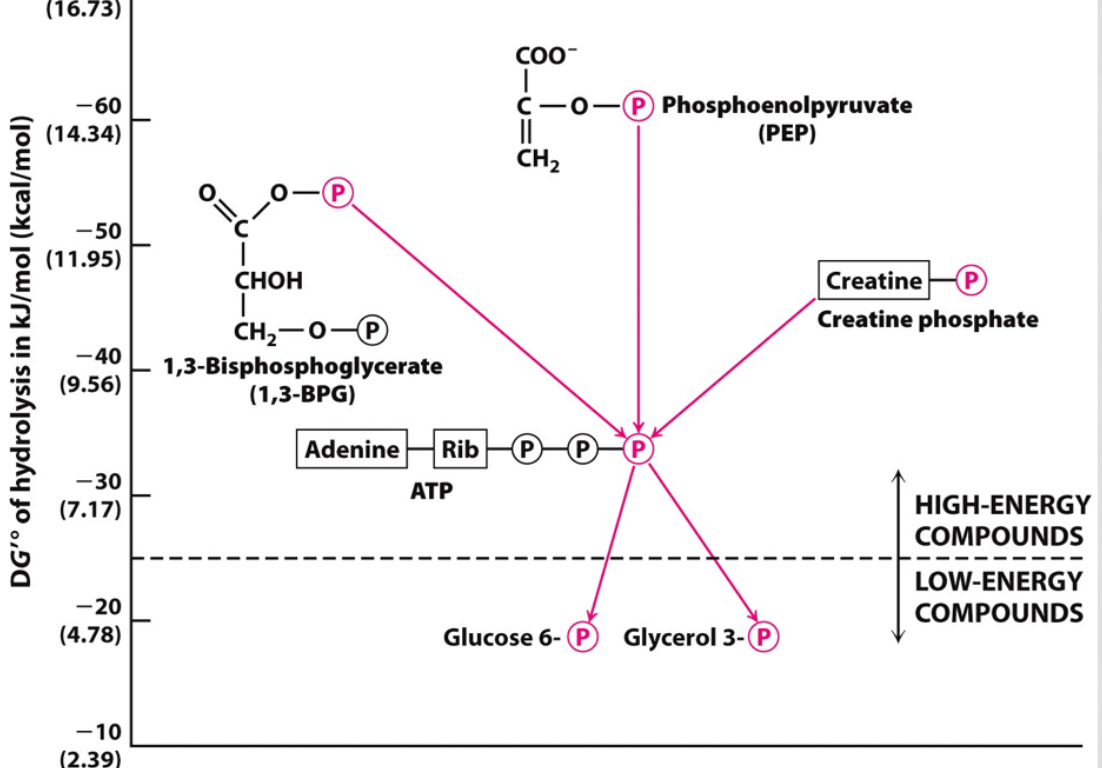
19
New cards
identify a step that shows substrate level phosphorylation
step 7-
1,3-bisphosphoglycerate --> 3-phosphoglycerate
and
ADP--> ATP
step 10-
phosphoenolpyruvate (PEP) --> pyruvate
and
ADP-->ATP
1,3-bisphosphoglycerate --> 3-phosphoglycerate
and
ADP--> ATP
step 10-
phosphoenolpyruvate (PEP) --> pyruvate
and
ADP-->ATP
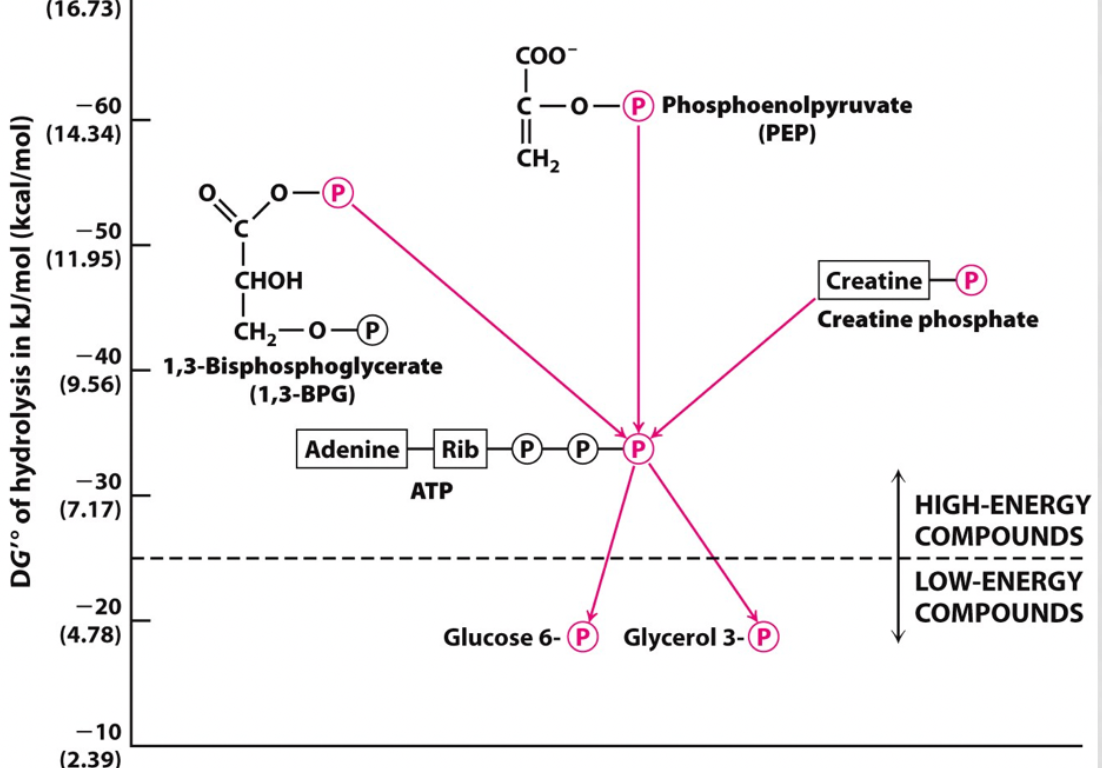
20
New cards
explain how coupling facilitates thermodynamically unfavorable reactions (ATP hydrolysis and oxidation driving les favorable reactions)
when one reaction is not thermodynamically favored it can be coupled with one that is greatly favored because delta Gs are additive and the excess negative delta G can cancel out the positive delta G
this means that the free energy released from the thermodynamically favored reaction can fuel the thermodynamically unfavorable reaction and still have excess that would make the whole coupled system favorable
ex. ATP is a high energy molecule with a high phosphoryl transfer potential as well as a decreased stability born out of the electrostatic repulsion of the three negatively charged phosphate groups next to each other. For this, ATP hydrolysis is an energetically favored reaction and is typically coupled with reactions like glucose phosphorylation to drive it
this means that the free energy released from the thermodynamically favored reaction can fuel the thermodynamically unfavorable reaction and still have excess that would make the whole coupled system favorable
ex. ATP is a high energy molecule with a high phosphoryl transfer potential as well as a decreased stability born out of the electrostatic repulsion of the three negatively charged phosphate groups next to each other. For this, ATP hydrolysis is an energetically favored reaction and is typically coupled with reactions like glucose phosphorylation to drive it
21
New cards
redox and thioester in glycolysis
oxidation is an energetically favored reaction as the removal of an electron leaves the molecule in a lower energy, more stable state
step 6 of glycolysis shows a redox reaction that uses a thioester as an intermediate to facilitate a coupled system. In the reaction, energy from the oxidation step is stored in the thioester intermediate which is a high-energy molecule which helps drive the unfavored step by providing a molecule which's reactivity is energetically favored and will drive the reaction to create the final product of 1,3-bisphosphoglycerate
step 6 of glycolysis shows a redox reaction that uses a thioester as an intermediate to facilitate a coupled system. In the reaction, energy from the oxidation step is stored in the thioester intermediate which is a high-energy molecule which helps drive the unfavored step by providing a molecule which's reactivity is energetically favored and will drive the reaction to create the final product of 1,3-bisphosphoglycerate
22
New cards
explain substrate level phosphorylation and why 1,3-bisphospglycerate and phosphoenolpyruvate are substrates
substrate level phosphorylation involves attaching a phosphate group to an ADP molecule to create ATP
both 1,3-bisphosphoglycerate and phosphoenolpyruvate are substrates of these reactions because they have a higher phosphoryl transfer potential than ATP and will readily give up a P to attach to ADP
both 1,3-bisphosphoglycerate and phosphoenolpyruvate are substrates of these reactions because they have a higher phosphoryl transfer potential than ATP and will readily give up a P to attach to ADP
23
New cards
PET scan and how it detects cancer
PET scans use slightly radioactive sugars that accumulate in areas of high glycolytic activity (as is common with cancerous tissue due to excess proliferation) and release gamma rays that are then detected with a machine. Since there is a lot of sugar accumulation in cancerous tissue, there is a stronger concentration of gamma rays being released from that location and will result in a bright spot in the PET scan
24
New cards
Warburg effect (glucose metabolism in normal cell v cancerous cell)
while normal cells typically use mitochondrial oxidative phosphorylation to generate ATP, cancerous cells rely on glycolysis even in the presence of oxygen which is why cancerous cells take up more of the radioactive sugar
25
New cards
Given the pathways of glycolysis and gluconeogenesis, identify which reactions are most energetically favorable in one pathway under typical physiological conditions, and thus must be bypassed in the opposing pathway
steps 1, 3, and 10 of glycolysis are thermodynamically favored to the point that it is considered irreversible. These steps must be bypassed during gluconeogenesis
26
New cards
identify the three ways that metabolic pathways are regulated
1. compartmentalization: controlling where the substrate is in relation to enzyme- product of one reaction is the substrate of next and much be transported to another organelle where the next enzyme is
2. regulation of gene expression: many points in the process for protein synthesis. ex. DNA transcription regulation; not as much enzyme created
3. regulation of enzyme activity: achieved through allosteric enzymes (R vs T (biologically inactive) states) and reversible covalent modification
2. regulation of gene expression: many points in the process for protein synthesis. ex. DNA transcription regulation; not as much enzyme created
3. regulation of enzyme activity: achieved through allosteric enzymes (R vs T (biologically inactive) states) and reversible covalent modification
27
New cards
describe the role of allosteric enzymes in the regulation of metabolic pathways
regulate the flux of biochemicals
forces enzymes in the T or R state based on regulation
forces enzymes in the T or R state based on regulation
28
New cards
PFK2/FBPase2 bifunctional enzyme pattern
serine phosphorylated= PFK2 inactive / FBpase2 active (enzyme dephosphorylates substrate and activates gluconeogenesis)
serine dephosphorylated = PFK2 active / FBPase2 inactive (enzyme phosphorylates substrate and activates glycolysis)
serine dephosphorylated = PFK2 active / FBPase2 inactive (enzyme phosphorylates substrate and activates glycolysis)
29
New cards
define flux and how flux of metabolic pathways is affected by allosteric regulation
all of the enzymes in a particular pathway will be affected equally by allosteric regulation - this causes a flux (consequential increase or decrease in pathway activity throughout the pathway)
allosteric regulation can be inhibitory or excitatory. If inhibitory, enzyme will be in the T phase while excitatory will set the enzyme in an R phase
for example, increased PFK2 will have an increased flux effect on glycolysis
allosteric regulation can be inhibitory or excitatory. If inhibitory, enzyme will be in the T phase while excitatory will set the enzyme in an R phase
for example, increased PFK2 will have an increased flux effect on glycolysis
30
New cards
R/T states; allosteric effect on RT
enzymes in equilibrium between relaxed (active) and tense (inactive) state
allosteric inhibition fixes enzyme in T state and allosteric activation fixes enzyme in R state
allosteric inhibition fixes enzyme in T state and allosteric activation fixes enzyme in R state
31
New cards
how and why AMP and ATP allosterically regulate flux of glycolysis and gluconeogenesis
ATP is the allosteric indicator of PFK1/1,6-FBPase activity
When ATP predominates, it attaches at the regulatory site and phosphorylates the serine which then causes an increased flux in gluconeogenesis by having 1,6-FBPase dephosphorylating fructose 1,6-bisphosphate
When AMP concentration predominates, the PFK1 section of the enzyme is activated which causes an increased flux in glycolysis and decrease in gluconeogenesis. This causes the dephosphorylated enzyme to start phosphorylating fructose 6-phosphate to 1,6-bisphosphate
When ATP predominates, it attaches at the regulatory site and phosphorylates the serine which then causes an increased flux in gluconeogenesis by having 1,6-FBPase dephosphorylating fructose 1,6-bisphosphate
When AMP concentration predominates, the PFK1 section of the enzyme is activated which causes an increased flux in glycolysis and decrease in gluconeogenesis. This causes the dephosphorylated enzyme to start phosphorylating fructose 6-phosphate to 1,6-bisphosphate
32
New cards
energy charge
[ATP] + 1/2 [ADP]
---------------------------
[ATP] + [ADP] + [AMP]
closer to 1 = ATP utilizing pathways
closer to 0 = ATP generating pathways
---------------------------
[ATP] + [ADP] + [AMP]
closer to 1 = ATP utilizing pathways
closer to 0 = ATP generating pathways
33
New cards
importance of reciprocal regulation of glycolysis and gluconeogenesis and role of allosteric enzymes in regulation
Both pathways are reciprocally regulated as to maintain blood sugar levels and energy substrate concentration within the cells
ATP works as a negative feedback indicator whether PFK1 should be activated to create more ATP or F1,6Bpase in an excess ATP environment to create glucose
ATP works as a negative feedback indicator whether PFK1 should be activated to create more ATP or F1,6Bpase in an excess ATP environment to create glucose
34
New cards
reciprocal enzyme activity of glycolysis and gluconeogenesis
when one pathway is highly active the other is inhibited; gluconeogenesis uses more ATP molecules than glycolysis produces so if both were to be activated at the same time, the net effect would be a depletion of ATP. Both glycolysis and gluconeogenesis are regulated by ATP concentration which acts an an allosteric regulator
Certain enzymes of the pathways are allosterically affected by both AMP and ATP or just one or the other. Regardless the flux they cause affects the entire pathway
Certain enzymes of the pathways are allosterically affected by both AMP and ATP or just one or the other. Regardless the flux they cause affects the entire pathway
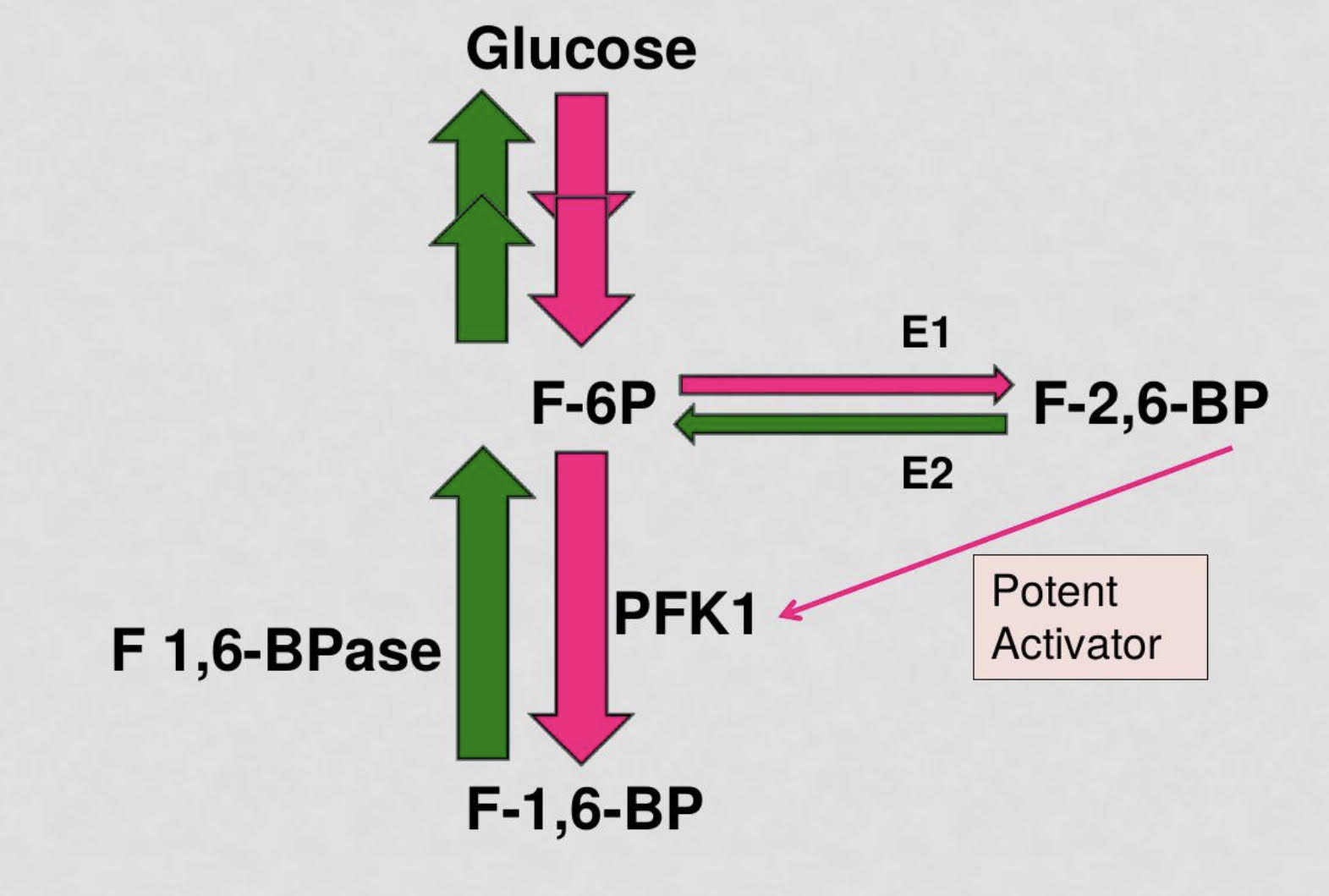
35
New cards
what are coenzymes
small organic cofactor derived from vitamins that support enzyme activity
36
New cards
biotin and gluconeogenesis
biotin binds to CO2 within gluconeogenesis
37
New cards
identify the steps of gluconeogenesis that are regulated by compartmentalization
Step 1: the first substrate that is used in gluconeogenesis, oxaloacetate, cannot pass through the mitochondria membrane so it is reduced into malate which can pass through the membrane through a malate protein channel and is then oxidized in the cytoplasm
steps in middle: occur in the cytoplasm
step 11: glucose is synthesized in the endoplasmic reticulum
steps in middle: occur in the cytoplasm
step 11: glucose is synthesized in the endoplasmic reticulum
38
New cards
compare and contrast the effect of insulin and glucagon on glucose metabolism in the fasting and fed states
fasting state: blood sugar level low --> increased glucagon (catabolic) --> glycolysis, glycogenolysis increased
fed state: blood sugar level high --> increased insulin (anabolic) --> gluconeogenesis, glycogen synthesis increased
vice versa
fed state: blood sugar level high --> increased insulin (anabolic) --> gluconeogenesis, glycogen synthesis increased
vice versa
39
New cards
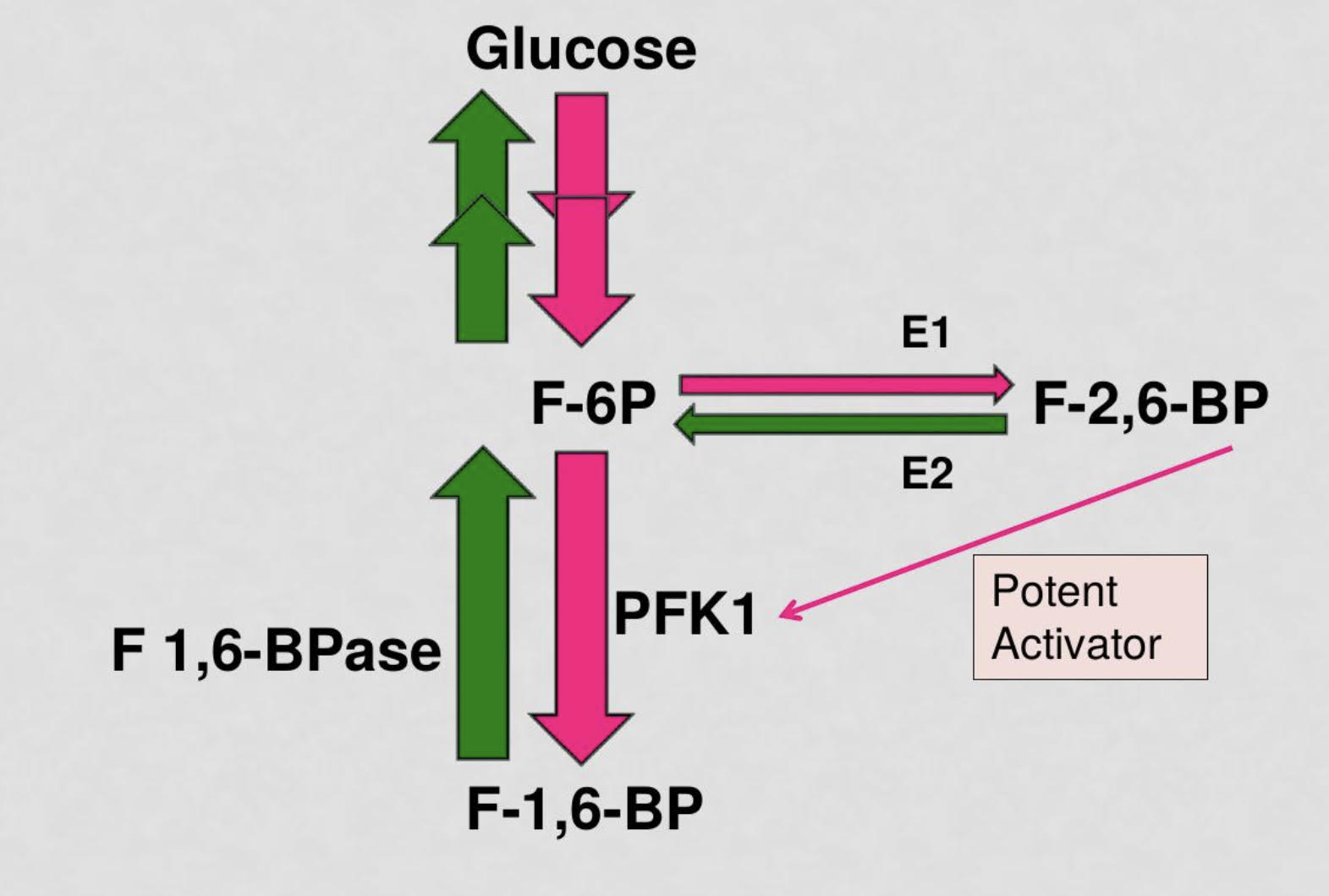
explain how bifunctional enzyme PFK2/FBPase2 controls the level of the PFK1 allosteric regulator fructose 2,6 bisphosphate. integrate with insulin/glucagon signaling
PFK2 uses some fructose-6-phosphate to create F-2,6-BP which is a potent activator of the enzyme PFK1 which facilitates glycolysis
FBPase2 is an inhibitory enzyme that reduces concentration of F-2,6-BP and increases activation of F-1,6-BPase which converts fructose-1,6-bisphosphate to fructose-6-ohosphate and facilitates gluconeogenesis
in states of high blood sugar levels, Insulin will be released and PFK2 will be activated to catalyze glycolysis PFK2 will use some fructose-6-phosphate to create fructose-2,6-bisphosphate which will highly catalyze the activity of PFK1 to turn fructose-6-phosphate to fructose-1,6-bisphosphate for glycolysis
in states of low blood sugar levels, glucagon will be released and FBPase2 will predominate and decrease the concentration of F 2,6 bpase and F1,6BPase will activate to catalyze gluconeogenesis
when the PKF2 domain of the bifunctional enzyme is phosphorylated, gluconeogenesis is activated due to the increased activity of fructose-1,6-bisphosphatase
when the PFK2 domain is not phosphorylated, glycolysis prevails as PFK2 is active
FBPase2 is an inhibitory enzyme that reduces concentration of F-2,6-BP and increases activation of F-1,6-BPase which converts fructose-1,6-bisphosphate to fructose-6-ohosphate and facilitates gluconeogenesis
in states of high blood sugar levels, Insulin will be released and PFK2 will be activated to catalyze glycolysis PFK2 will use some fructose-6-phosphate to create fructose-2,6-bisphosphate which will highly catalyze the activity of PFK1 to turn fructose-6-phosphate to fructose-1,6-bisphosphate for glycolysis
in states of low blood sugar levels, glucagon will be released and FBPase2 will predominate and decrease the concentration of F 2,6 bpase and F1,6BPase will activate to catalyze gluconeogenesis
when the PKF2 domain of the bifunctional enzyme is phosphorylated, gluconeogenesis is activated due to the increased activity of fructose-1,6-bisphosphatase
when the PFK2 domain is not phosphorylated, glycolysis prevails as PFK2 is active
40
New cards
signal, receptor, signaling effectors, and short/long term effects in insulin signaling pathway
**application question
**application question
signal: insulin
receptor: tyrosine kinase
signaling effectors: kinase cascade (signal amplification)
short term effect: cellular movement OR enzyme activation
long term effect: altered DNA transcription
receptor: tyrosine kinase
signaling effectors: kinase cascade (signal amplification)
short term effect: cellular movement OR enzyme activation
long term effect: altered DNA transcription
41
New cards
kinases v phosphatases
kinases - phosphorylate (Take P from ATP/GTP and attach it to substrate)
phosphatases- dephosphorylates (takes P from substrate and removes it w water molecule)
phosphatases- dephosphorylates (takes P from substrate and removes it w water molecule)
42
New cards
how does insulin signaling pathway affect GLUT4 location in the cell
a messenger protein released from the insulin receptor complex separates and activates cellular movement by taking vesicles with GLUT4 transports to the cell membrane and inputs the GLUT4 channels into the cells membrane
cytoplasm --> cell membrane
cytoplasm --> cell membrane
43
New cards
Glycolysis and gluconeogenesis are reciprocally regulated. The committed step in glycolysis, the conversion of fructose 6-phosphate to fructose 1,6-bisphosphate is catalyzed by the enzyme (PFK1/fructose 1,6 bisphosphatase). This type of enzyme is a (kinase/phosphatase) and its function is to phosphorylate its substrate, using ATP as the phosphoryl donor. This enzyme's activity is greatly stimulated when (fructose 6 phosphate/fructose 1,6 bisphosphate/fructose 2,6 bisphosphate)
binds to an allosteric site as a potent activator, resulting in increased flux through glycolysis. Two enzymes control the amount of this molecule in the cell. (PFK1/PFK2/FBPase2/ F1,6 BPase) is the enzyme that makes it, by phosphorylating fructose 6-phosphate. It does not phosphorylate all of fructose 6-phosphate: only a little because this is such a potent activator. When glycolysis needs to slow and gluconeogenesis predominates, then this enzyme is active: (PFK1/PFK2/FBPase2/F1,6BPase), dephosphorylating this potent activator back to fructose 6-phosphate.
binds to an allosteric site as a potent activator, resulting in increased flux through glycolysis. Two enzymes control the amount of this molecule in the cell. (PFK1/PFK2/FBPase2/ F1,6 BPase) is the enzyme that makes it, by phosphorylating fructose 6-phosphate. It does not phosphorylate all of fructose 6-phosphate: only a little because this is such a potent activator. When glycolysis needs to slow and gluconeogenesis predominates, then this enzyme is active: (PFK1/PFK2/FBPase2/F1,6BPase), dephosphorylating this potent activator back to fructose 6-phosphate.
PFK1
kinase
fructose 2,6 bisphosphate
PFK2
FBPase2
kinase
fructose 2,6 bisphosphate
PFK2
FBPase2
44
New cards
explain type 1 diabetes with insulin signaling pathway
type 1 diabetes is a autoimmune disease that is caused due to a decreased production of insulin from pancreatic cells
because of the lack of insulin, the signaling pathway within the cells is decreased meaning that GLUT4 channels are not placed into the cell membrane with required efficiency so sugar tends to stay in the blood stream
because of the lack of insulin, the signaling pathway within the cells is decreased meaning that GLUT4 channels are not placed into the cell membrane with required efficiency so sugar tends to stay in the blood stream
45
New cards
identify the subcellular locations where each step of glycolysis and gluconeogenesis occur in a liver cell. integrate both pathways
all of glycolysis occurs in the cytoplasm
step 1 of gluconeogenesis occurs in the mitochondrial matrix
step 2-10 of gluconeogenesis occurs in the cytoplasm (overlap with glycolysis- think bifunctional enzyme for fructose-6-phosphate
step 1 of gluconeogenesis occurs in the mitochondrial matrix
step 2-10 of gluconeogenesis occurs in the cytoplasm (overlap with glycolysis- think bifunctional enzyme for fructose-6-phosphate