Optical isomerism part 1
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
24 Terms
what is optical isomerism ?
Optical isomerism is a type of steroisomerism.
Optical isomers have a chiral carbon atom.
What is a stereoisomers ?
Stereoisomers have the same structural formula, but have their atoms arranged differently in space.
what can optical isomers or enantiomers arranged to ?
It can arrange to groups in 2 different ways around the chiral carbon atoms so that 2 different molecules are made.
what can enantiomers turn to and not turn to ?
enantiomers are mirror image and no matter which way you turn them they cannot be superimposed.
what can no optical isomers turn to and not turn to ?
molecules are or can be superimposed , they are achiral.
what does the term racemate / racemic mixture mean ?
it means that its a 50 / 50 of the enantiomers.
is propan - 2 ol optically active ? Draw the displayed formula
no its not Ch3 - CH ( OH ) - CH3
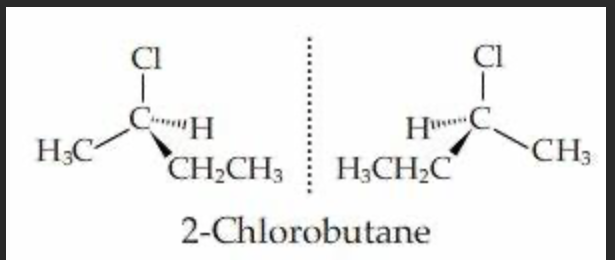
Is 2 - chlorobutane optically active. Draw the displayed formula + isomers
yes it is CH3 - CH ( CI ) - CH2 - CH3
is 1 - chlorobutane optically active ? Draw the displayed formula + isomers
no its not CH2 ( CI ) - CH2 - CH2 -CH3
is 3 - methyl hexane optically active ? Draw the displayed formula + isomers
yes it is. CH3 - CH2 - CH ( CH3 ) - CH2 - CH2 - CH3
is butanone optically active ? Draw a displayed formula + isomers
no its not CH3 - CH2 - CO - CH3
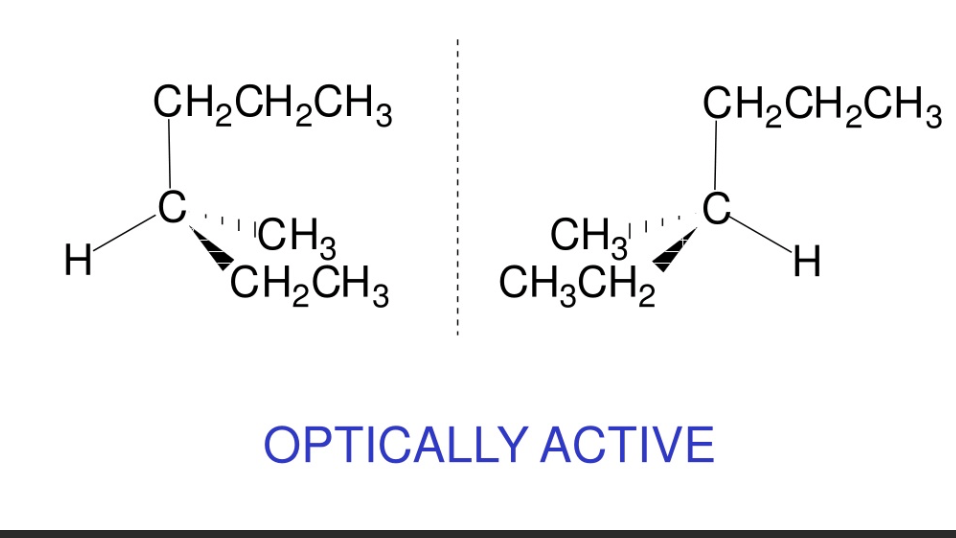
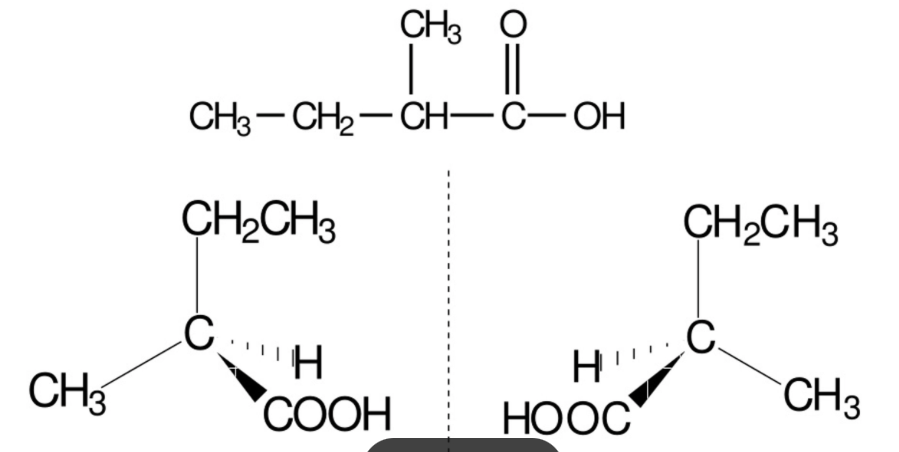
is 2 - methlybutanoic acid optically active ? Draw a displayed formula + isomers
yes it is CH3 - CH2 - CH ( CH3 ) - CO - CH3
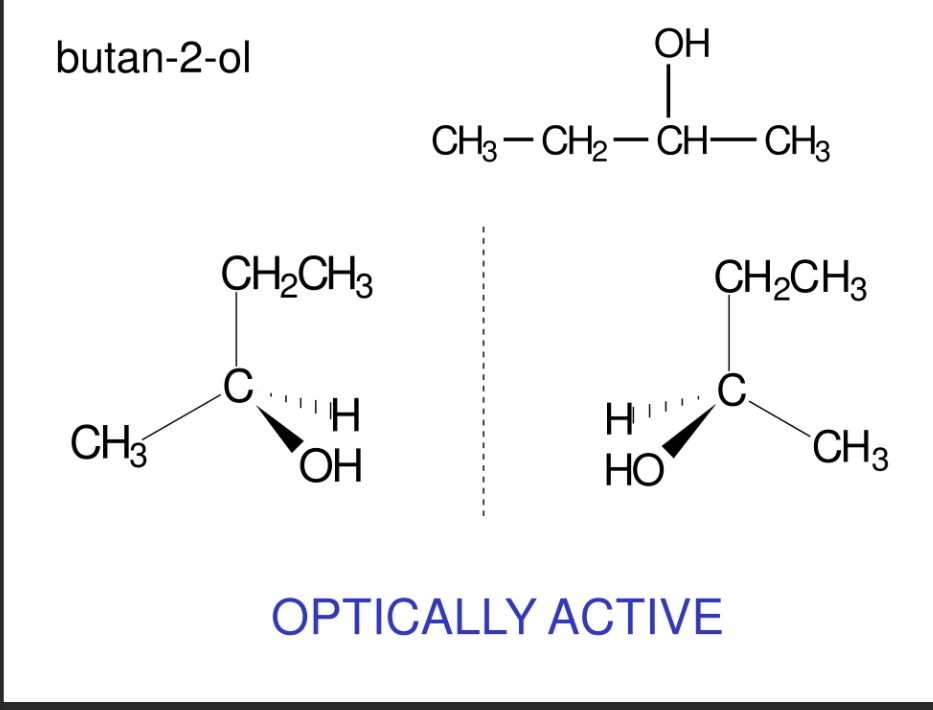
Is butan - 2 ol optically active ? Draw a displayed formula + isomers
yes it is CH3 - CH2 - CH ( OH ) - CH3
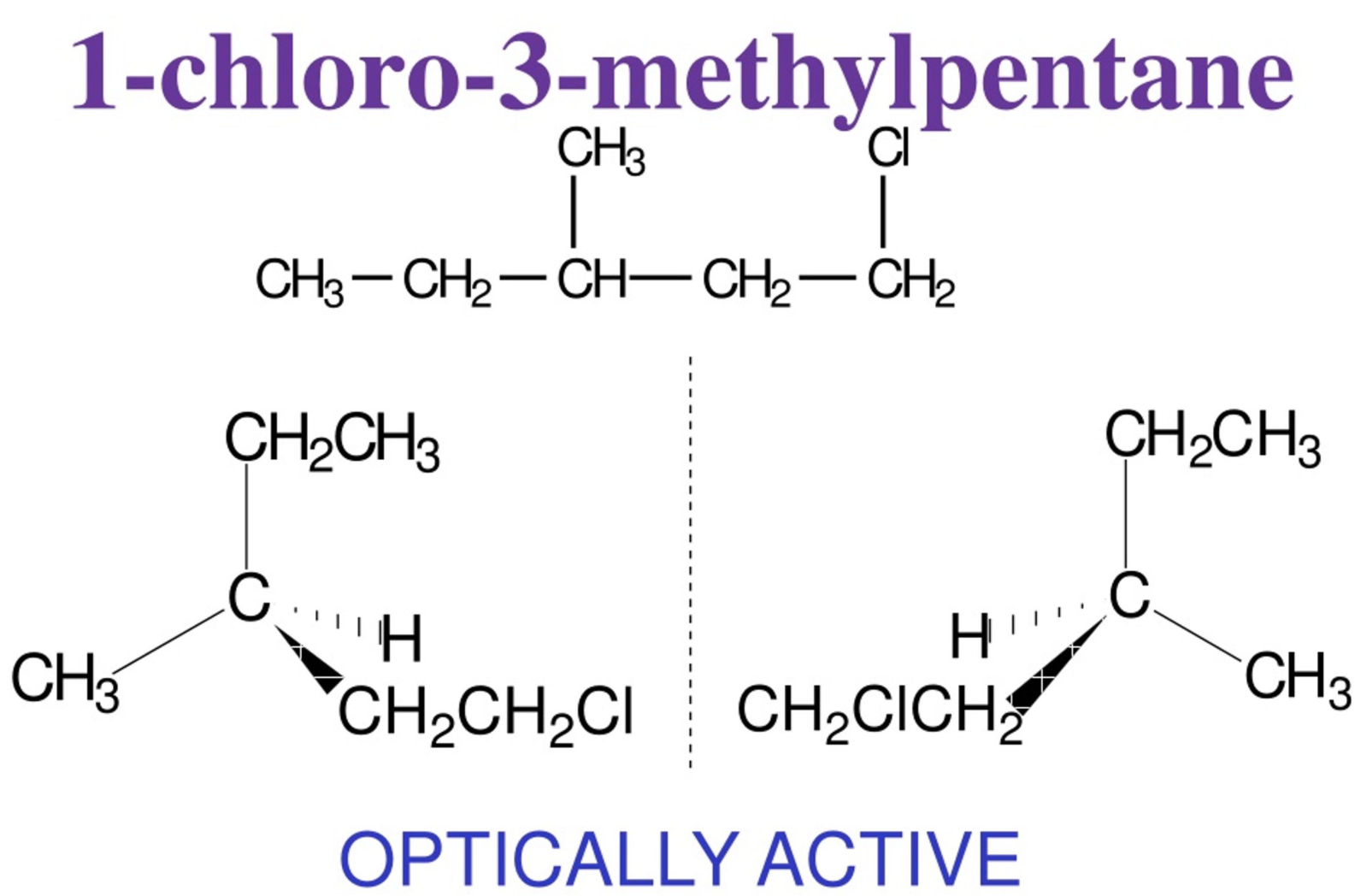
Is 1 - Chloro - 3 methylpentane optically active ? Draw a displayed formula + isomers
Yes it is CH3 - CH2 - CH ( CH3 ) - CH2 - CH2 CI
Is propan - 1 ol optically active ? Draw a displayed formula + isomers
no its not , CH3 - CH ( OH ) -CH3
What are the properties of enantiomers ?
Have identical chemical and physical properties except
Their effect on plane polarised light & their reaction with other chiral molecules
what radiation is light ?
Light is a form of electromagnetic radiation
the wave vibration are perpendicular to the direction of travel of the wave.
Optical isomers rotate the plane polarised light.

Chiral molecules often react same or differently with other chiral molecules ?
Differently
Most and many natural molecules are ?
Chiral ( 4 different groups ) attached to a carbon + are affected by optical isomers
what is an example of chiral molecule ?
Amino acidS ( proteins ) are chiral , along with many other molecules.
In nature only 1 optical imposers occurs eg : all natural amino acid rotate polarised light to the left
Is most drugs optically active ?
Yes, with only 1 enantiomer having the beneficial effect.
However some drugs, the other enantiomers can he harmful.