Chemistry AQA GCSE C.10 - Using Resources
1/39
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
40 Terms
Define a Finite resource
a resource that is being used up at a faster rate then they can be replaced - eventually they'l run out
Examples of finite resources
metal ores, crude oil, limestone
soda-lime glass
The most common type of glass. Its main ingredients include sodium carbonate, soda, and lime.
Define renewable resource
Resources normally replaced by natural processes and aren't depleted by moderate use
Examples of renewable resources
solar energy, biological resources such as forests & fisheries, biological organisms and crops that are used to make biofuels
describe the general structure of a composite
materials that consist of a reinforcement (aka. fibres) that's surrounded by a matrix or a binder
give an example of renewable resources in plastic industries and explain why
Plastic industries use ethane to produce polymers which is normally made from crude oil. Ethane can be made from ethanol - fermenting glucose - allowing the industry to conserve the finite resources and improve sustainability
bronze
a mixture of copper and tin
brass
An alloy of copper and zinc.
corrosion
the deterioration of a metal due to a chemical reaction in the environment
give an example of a synthetic product that's replaced or used in addition to a natural resource
poly(ester) is used instead of cotton
how do chemists play a role within sustainable development? explain using an example (2)
they can develop and adapt processes that use less resources.
.e.g. development of catalysts reduce amount of energy required for industrial processes.
Define potable water
Water that humans can safely drink
What is potable water?
water that's not 100% H20 as other ions .e.g. F-, Na+, Ca2+, giving the water it's taste
What's distillation?
purifying a liquid through either heating or cooling
give 3 things that can be used to sterilise fresh water
chlorine, ozone and ultraviolet light
describe the process of how the water treatment process occurs
- Water enters the water treatment through a barred screen which catches any large objects .e.g. twigs/leaves
- The water goes into a settlement tank where the sand and soil is settled out
- Aluminium sulphate and lime are added to the water causing small dirt particles to clump together so they sink to the bottom.
- Water is passed through a filter made of fine sand and gravel which removes remaining particles .e.g. mud or grit
- A small amount of chlorine is added to kill harmful bacteria in the water (ozone can be added or UV light used)
- The water's pH is checked and corrected to neutral and is then stored in large tanks ready to be pumped out
desalnation
purifying salty water into potable water
describe the process of distillation
1) measure 20cm^3 salt water into conical flask
2) make sure conical flask is held on tripod and gauze using a clamp stand
3) place ice water in a beaker surrounding the test tube
4) heat the water with a Bunsen burner until it starts to boil
5) reduce the heat so the water gently boils
6) the water will evaporate and condense into the cooled test tube
7) collect around 1cm^3 of potable water and stop heating the salt water
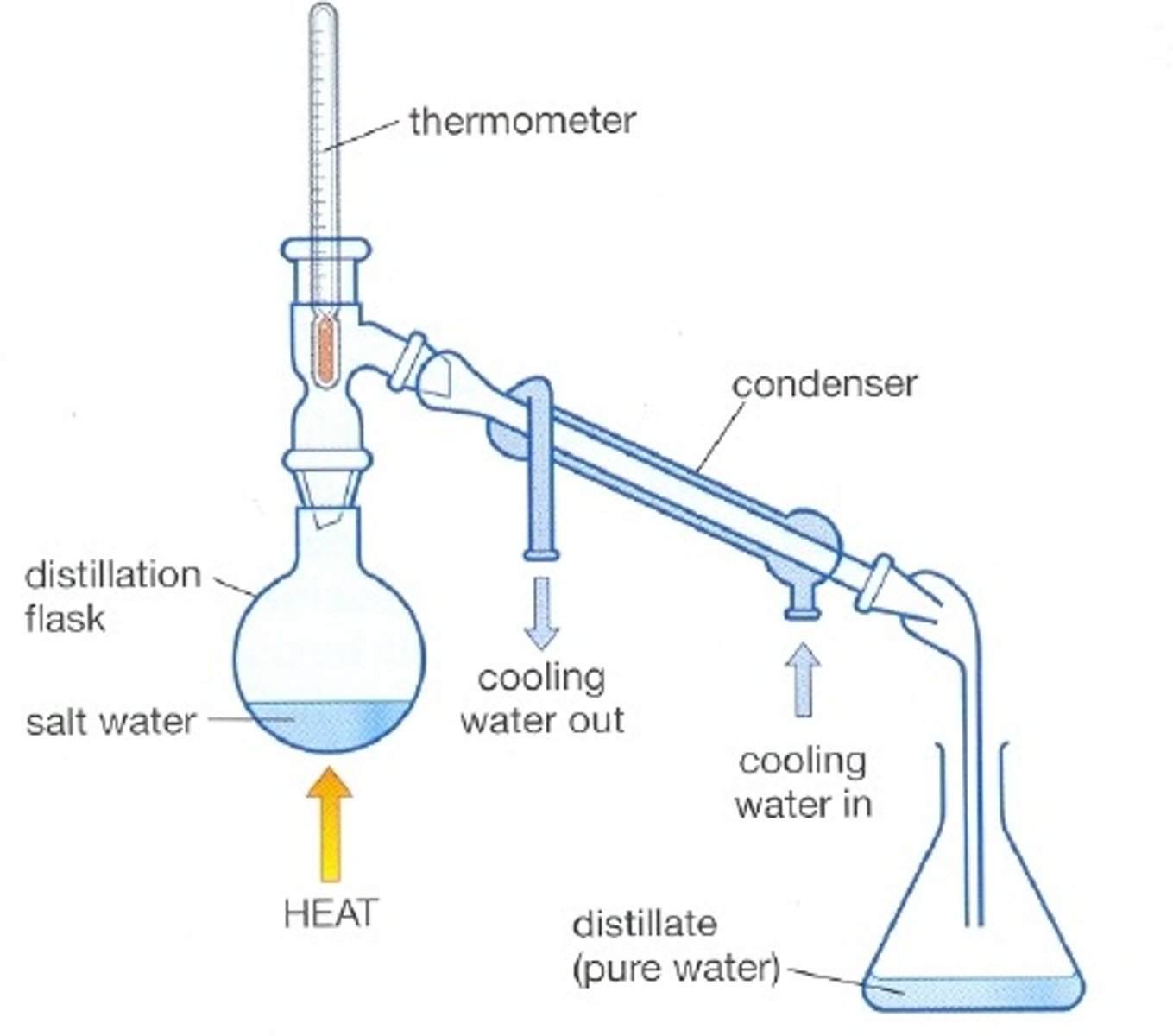
explain why we don't use desalination to produce large quantities of potable water in the UK (2)
Lots of energy is used for desalination compared to filtration and sterilisation of fresh water.
Since the UK has a plentiful supply of fresh water, there's no need to use the desalination process.
how to test a salt
sodium = a yellow flame test
chlorine = white precipitate with silver nitrate
describe the process of sewage treatment
1) Screening - moves past a metal grid that removes large objects and grit from the waste water
2) Primary treatment - solid sediments are allowed to settle out from the mixture. Paddles rotate which causes sludge to be pushed towards the centre of the tank
3) Secondary treatment - Useful bacteria feed on remaining organic matter breaking them down aerobically
4) Final treatment - Sediment is either recycled back to secondary treatment tank or passed into sludge treatment. Waste water is now safe enough to be discharged into rivers
treating sewage sludge
Digested anaerobically by microorganisms beneath the tank's surface at around 55 degrees C or as low as 35 degrees C but can take up to 30 days to complete. Energy has to be supplied to heat the sludge
Breakdown products = biogas (methane + carbon dioxide + hydrogen sulphide) which can be burned to power the treatment plant or provide electricity.
Sludge can be dried out into fertiliser or used as a renewable energy source
Bioleaching
Process of extraction of metals from ores using microorganisms
Phytomining
The process of extraction of metals from ores using plants
Life Cycle Assessment (LCA)
A technique to assess environmental impacts associated with all the stages of a product's life, i.e., from raw material extraction through materials processing, manufacture, distribution, use, repair and maintenance, and disposal or recycling. While typically used to assess environmental impacts, this technique could be used to identify social impacts of a product's life cycle as well
Stages of Product
raw material, manufacture, use, maintenance, disposal
what needs to be considered in a LCA?
- resources used
- energy use
- disposing after use
- impact on environment
how to carry out an LCA
- list all energy + material inputs and all outputs into the environment
- evaluate the potential environmental impacts from these inputs and outputs
- interpret the results to help compare/make decisions about products, material, process or service
what is an LCA useful for?
- comparing materials for the same job
- promoting sustainable resources
describe the process of recycling aluminium
- fed on a conveyor belt which then shreds the metal and decoates it
- aluminium is then melted in a furnace and into a tilting holding furnace
- It's then put in a filtration unit
- Direct chill + casting unit and then shredded again into ingots (large sheets of aluminium)
explain why recycling materials is better for the environment
- It reduces the exploitation of the Earth's limited resources
- It saves lots of energy and therefore saves money
- The pollution caused by mining and extraction of metals is also reduced by recycling since it's a reuse of the material
suggest why burning wood instead of coal would help reduce our carbon footprint (3)
we can replant the trees used and the CO2 in the atmosphere can be used for photosynthesis. No new CO2 would be produced as the CO2 in the trees would have been absorbed millions of years ago.
explain how green plants and other organisms have changed the composition of the Earth's atmosphere (4)
Plants and organisms photosynthesise using sunlight and CO2 to make glucose and oxygen and when they die, they trap CO2 in rocks and fuels. Nitrogen gas is produced as methane and ammonia react with oxygen, this forms the ozone layer.
based on Le Chatelier's principle, predict whether the reaction of the Haber process should be performed at high or low temperatures to achieve maximum yield. (3)
Low temperature because the point of equilibrium shifts in favour of the forward reaction which means more ammonia is produced.
state the conditions of the Haber Process
450 degreesC
200 atmospheres
anaerobic conditions
name 2 compounds of potassium that are used to make NPK fertilisers
potassium chloride
potassium sulfate
phosphate rock + nitric acid/sulfuric acid -->
souble salt that can be used in fetilisers
NPK fertilisers contain
- Nitrogen (N) (in the form of nitrate ions) for growth of leaves and stems
- Phosphorus (P) ( in the form of phosphate ions) for healthy roots
- Potassium (K) ( in the form of potassium ions) for healthy leaves and flowers
describe the difference between borosilicate glass and soda lime glass
Borosilicate Glass is Much More Resistant to Thermal Shock. Borosilicate glass has a very low CTE (Coefficient of Thermal Expansion) whereas soda lime glass has a very high CTE. It's for this reason that all quality cookware is made from borosilicate glass.