Protein Three-Dimensional Structure - Chapter #4 Biochemistry
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
25 Terms
Primary Structure
- Amino Acids Are
Linked by Peptide Bonds to Form Polypeptide
Chains
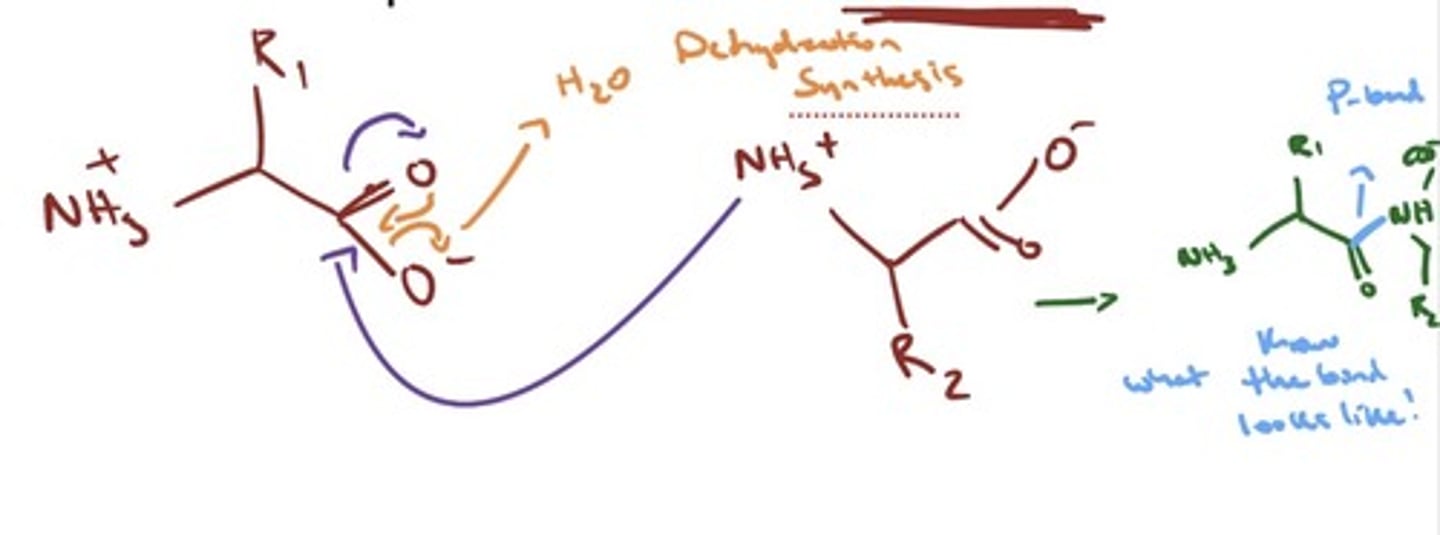
- Polypeptides consist of amino acids linked by a peptide bond
- The peptide bond is also called an amide bond
- Each amino acid in a protein is called a residue
- dehydration synthesis achieves this bond
Peptide Bond Formation
- get rid of O and two hydrogens from N
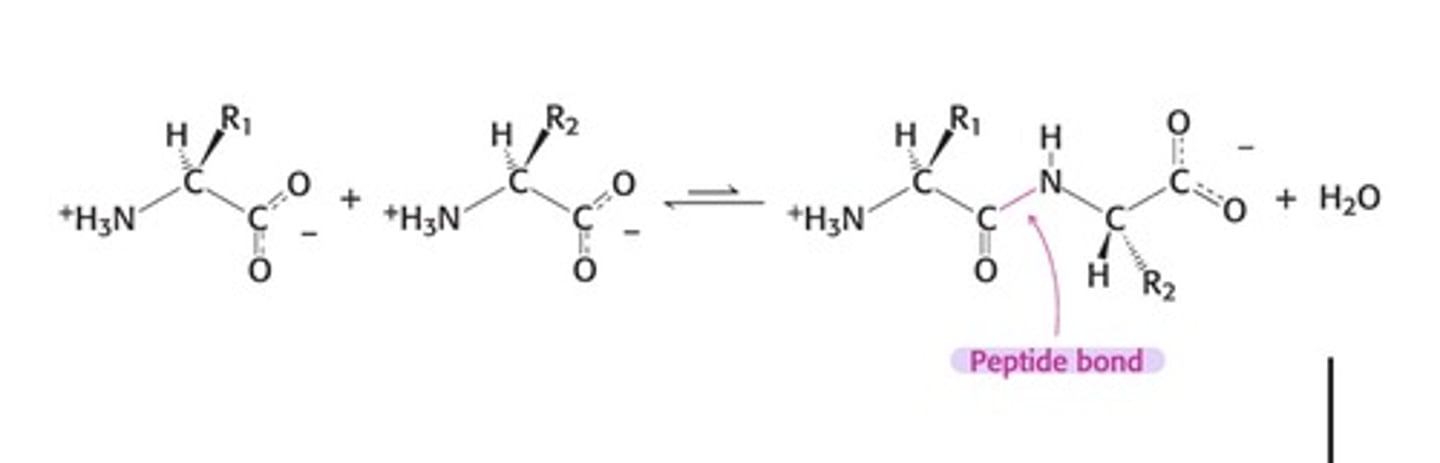
Polypeptide Chains Have Directionality - what group is at the beginning and at the end
- The amino terminal (N-terminus) end is taken as the beginning of the polypeptide chain, and the Carboxyl terminal (C-terminus) end is the end of the polypeptide chain
- N on the left and C on the right
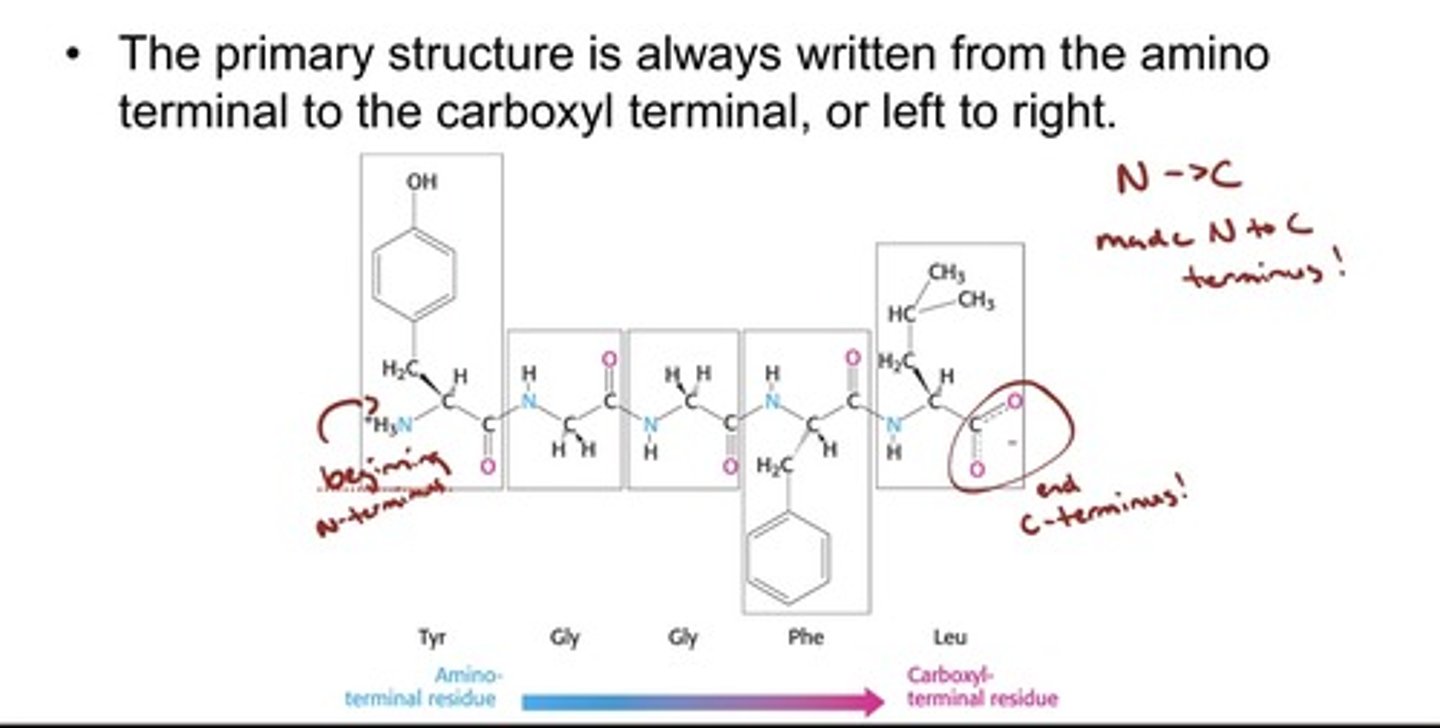
Backbones of polypeptides
- the polypeptide chain consists of a repeating part called
the main chain or backbone and a variable part consisting
of the distinctive amino acid side chains (R-groups).
- The backbone has hydrogen-bonding potential
because of the carbonyl groups and hydrogen
atoms that are bonded to the nitrogen of the
amine group
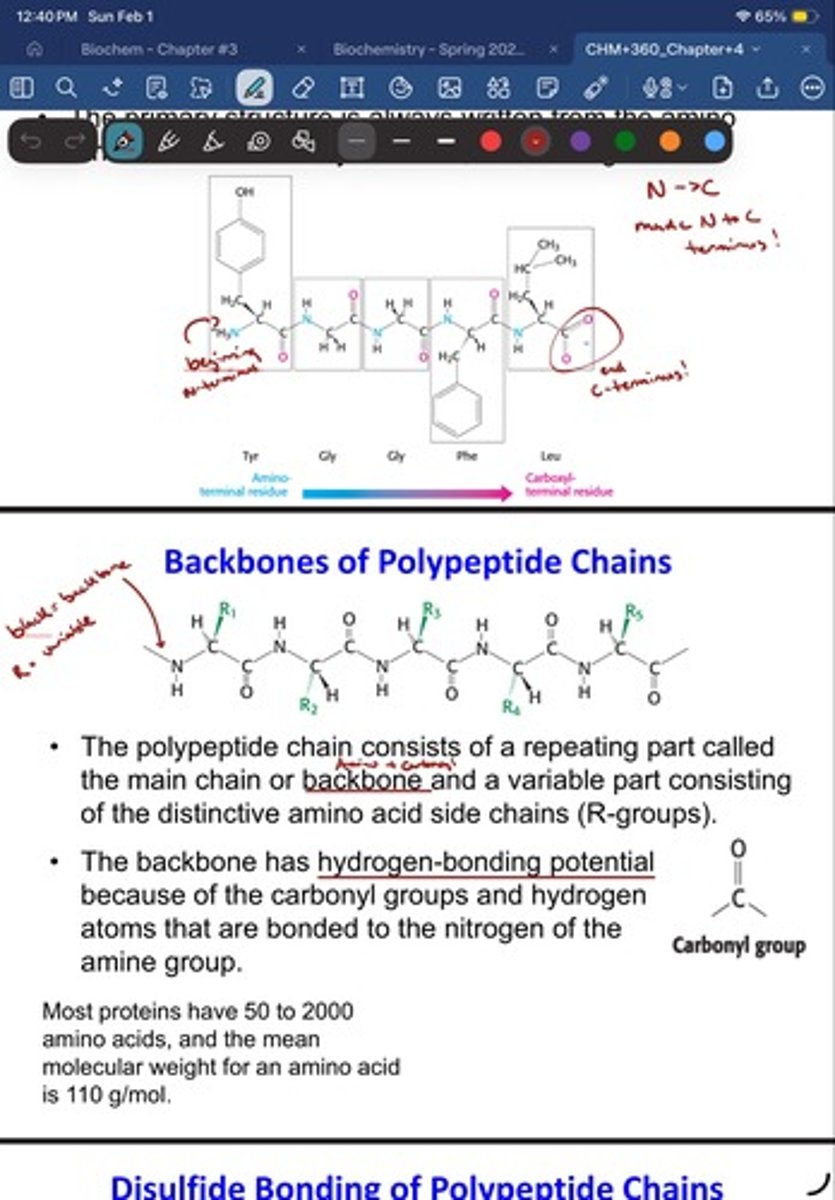
Disulfide Bonding of Polypeptide Chains
• In some proteins, the polypeptide chain can be cross-
linked by disulfide bonds.
• Disulfide bonds form by the oxidation of two
cysteines
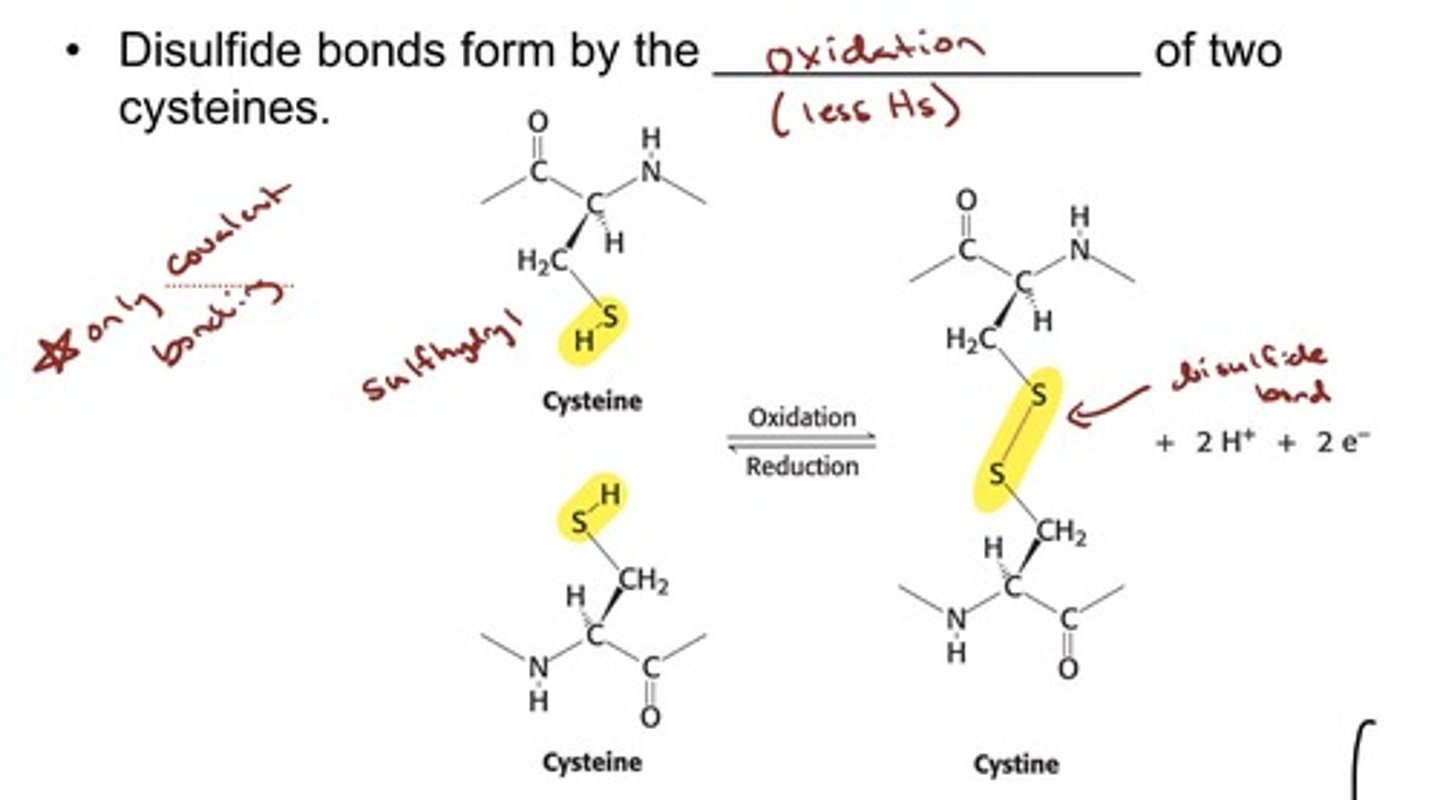
Proteins Have Unique Amino Acid Sequences
Specified by Genes - Inter and Intra bonds of cysteine
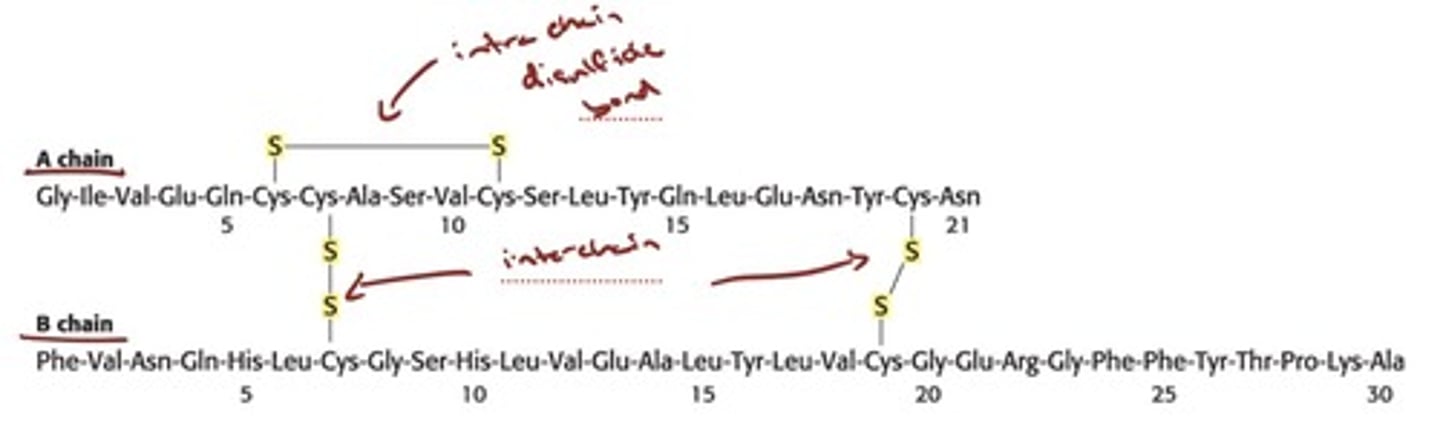
Polypeptide Chains Are Flexible Yet
Conformationally Restricted
- The peptide bond has partial double-bond character
because of resonance; thus, rotation about the bond
is prohibited.
- knwo either form of peptide bond!
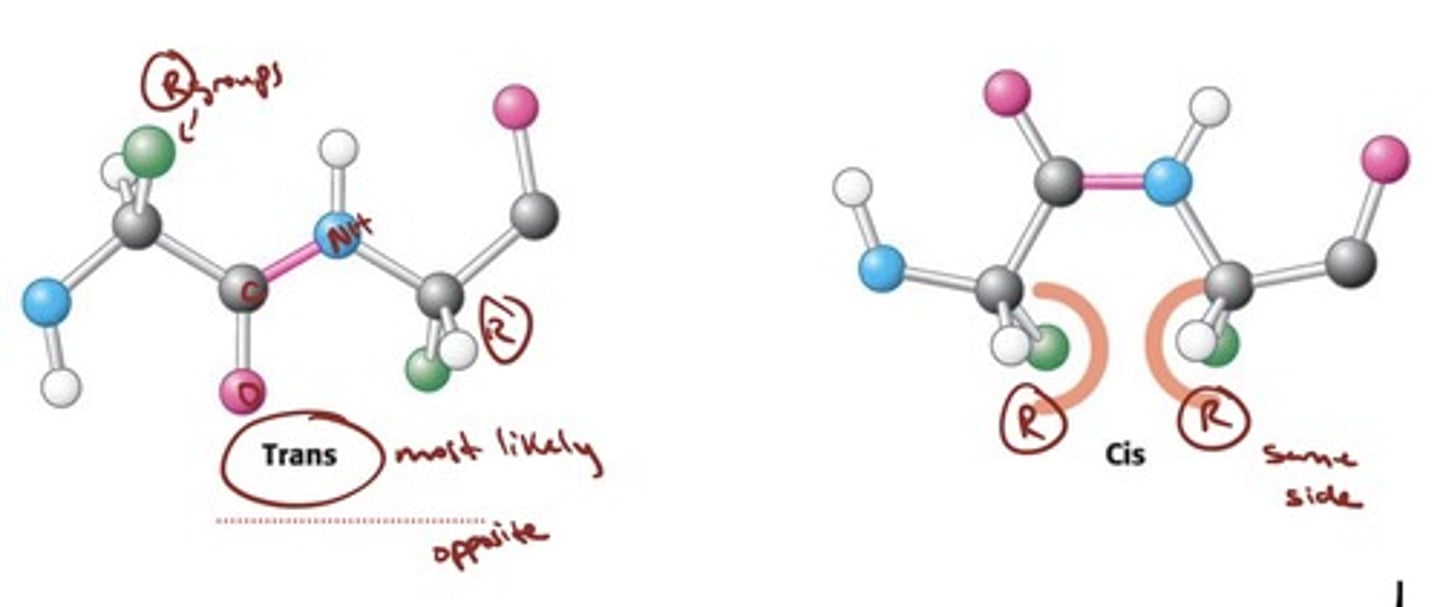
trans VS cis for AA chains
- Most peptide bonds are in the trans configuration so as
to minimize steric clashes between neighboring R
groups.
Secondary Structure
- Secondary structure is the three-dimensional structure
formed by hydrogen bonds between peptide NH
and CO groups of amino acids that are near one another in
the primary structure
- The α helix, β sheets, and turns are prominent examples
of secondary structure
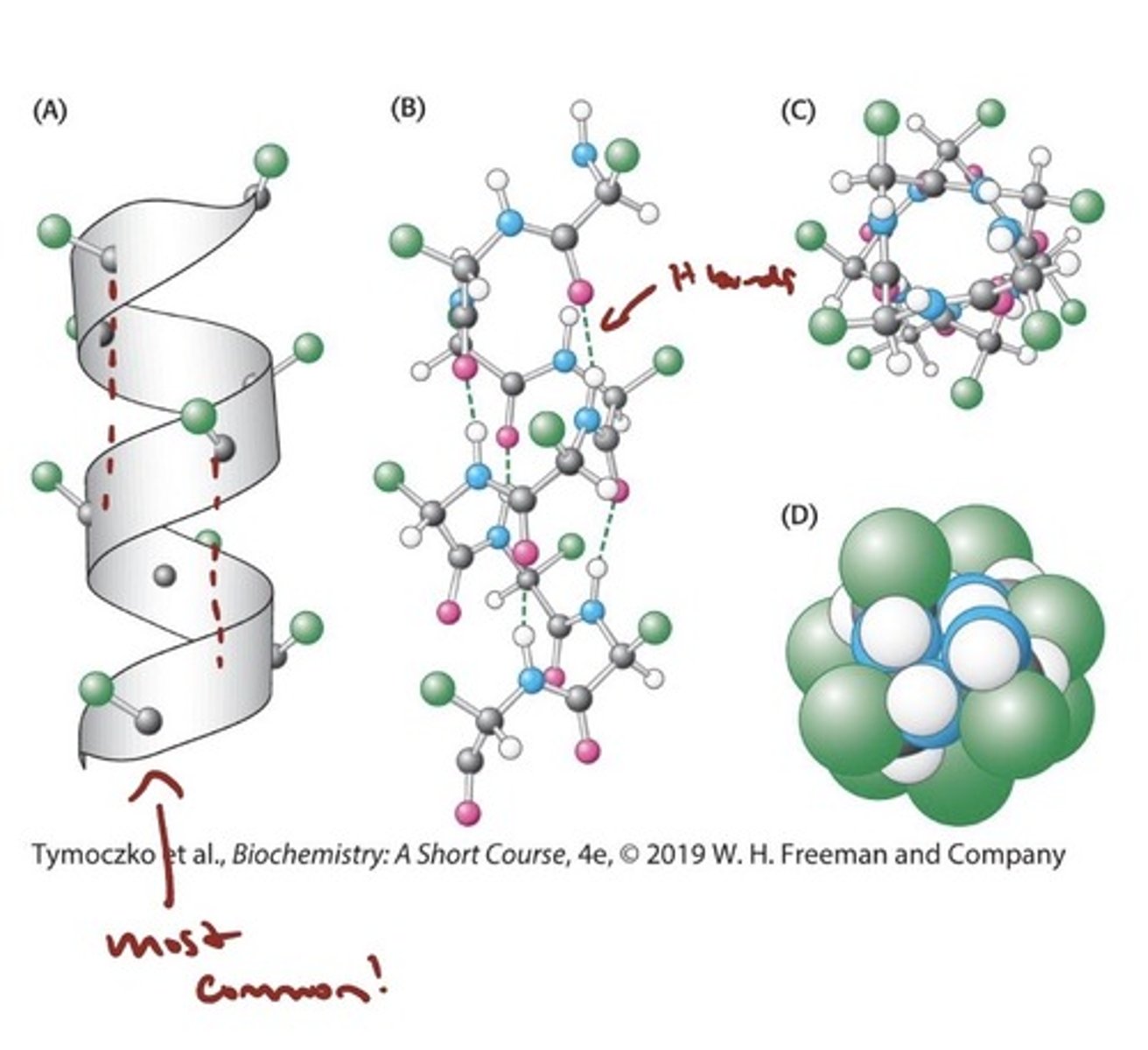
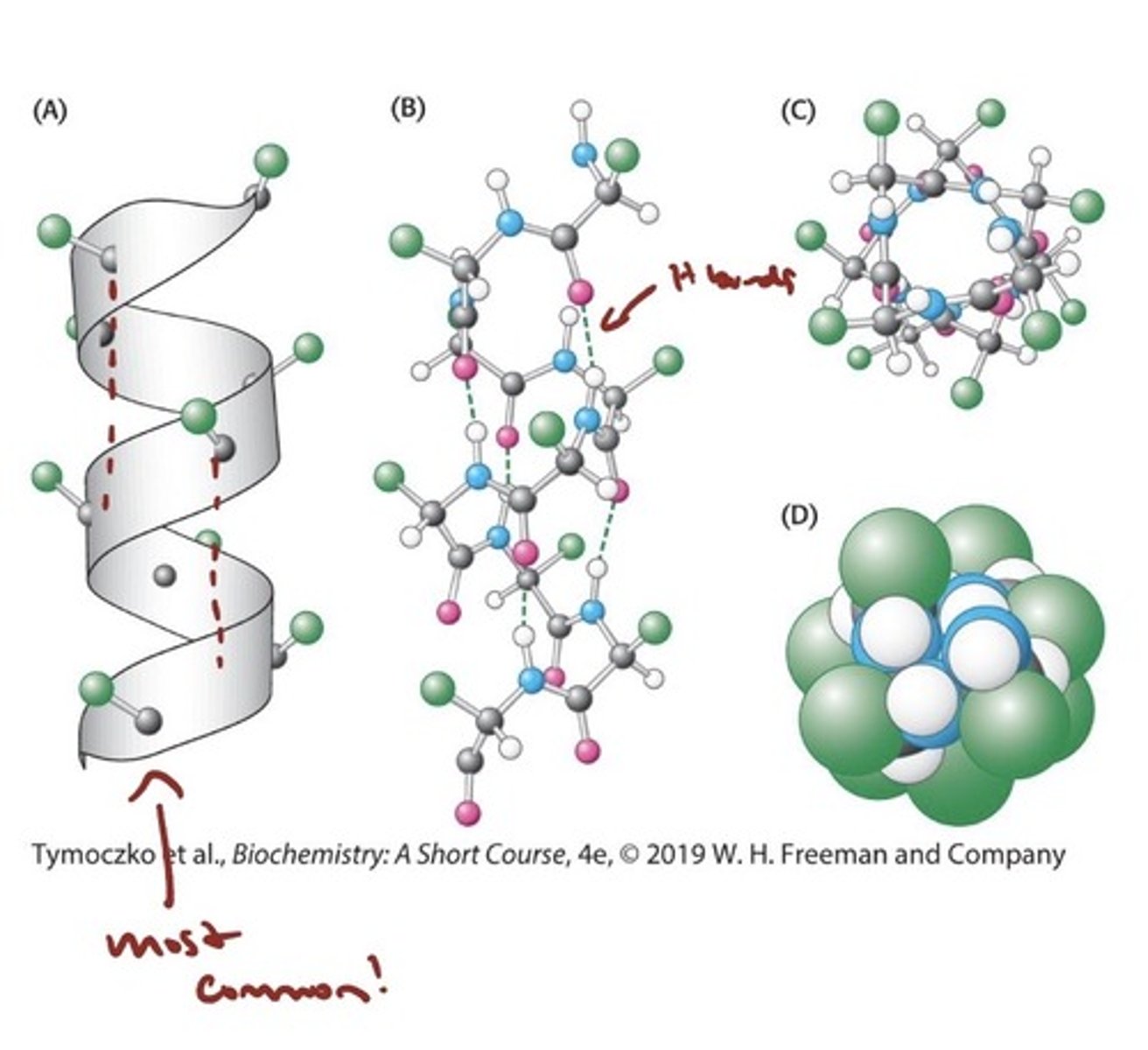
The Alpha Helix
- a Coiled Structure Stabilized
by Intrachain Hydrogen Bonds
- The α helix is a tightly coiled structure, with the R groups
bristling out from the axis of the helix
- The CO group of each amino acid forms a hydrogen bond with the NH group of the amino acid that is situated four residues ahead in the sequence (i + 4)
- All of the backbone CO and NH groups form hydrogen bonds except those at the end of the helix.
- all found are right handed
The Hydrogen-Bonding Scheme for an Alpha
Helix - what destabalizes it?
- Val, Thr, and Ile (steric clashes)
- Ser, Asp, and Asn (H-bond
donor/acceptor)
- Pro (lacks NH group, ring structure
prevents bond rotation)* really messed them up*
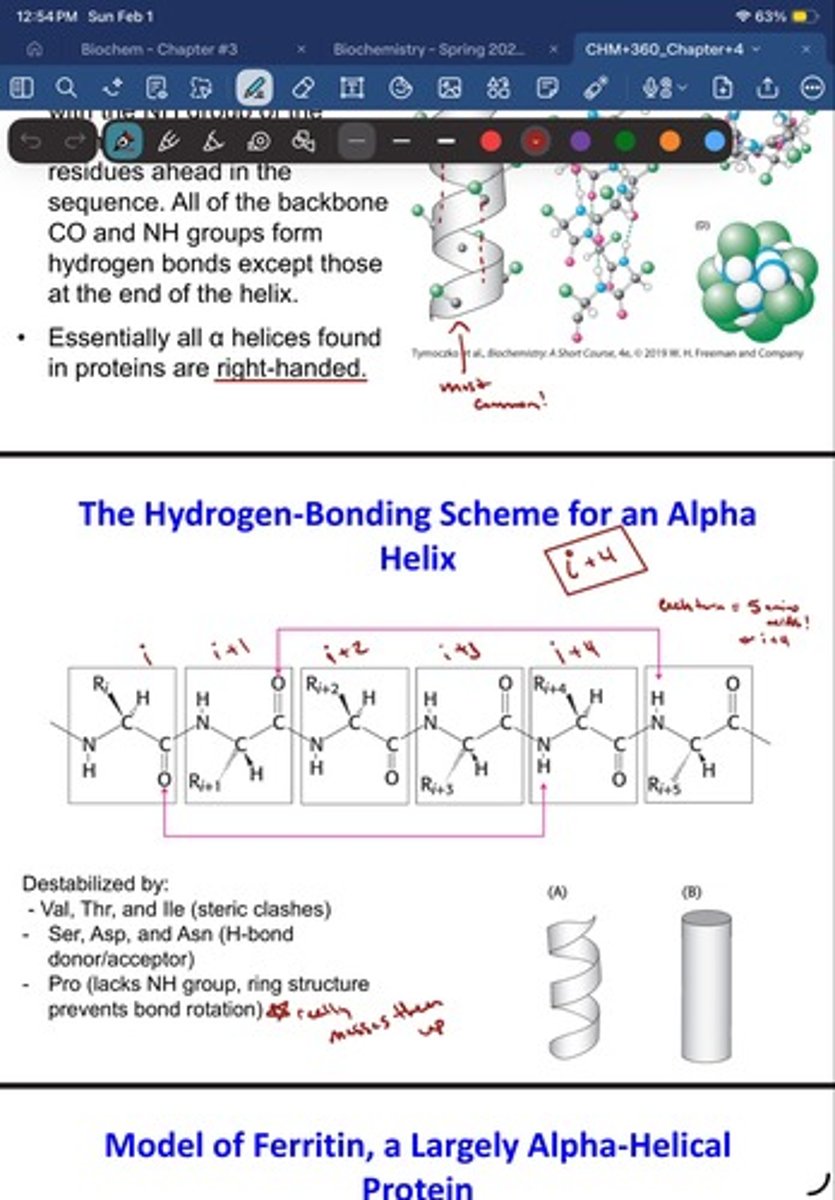
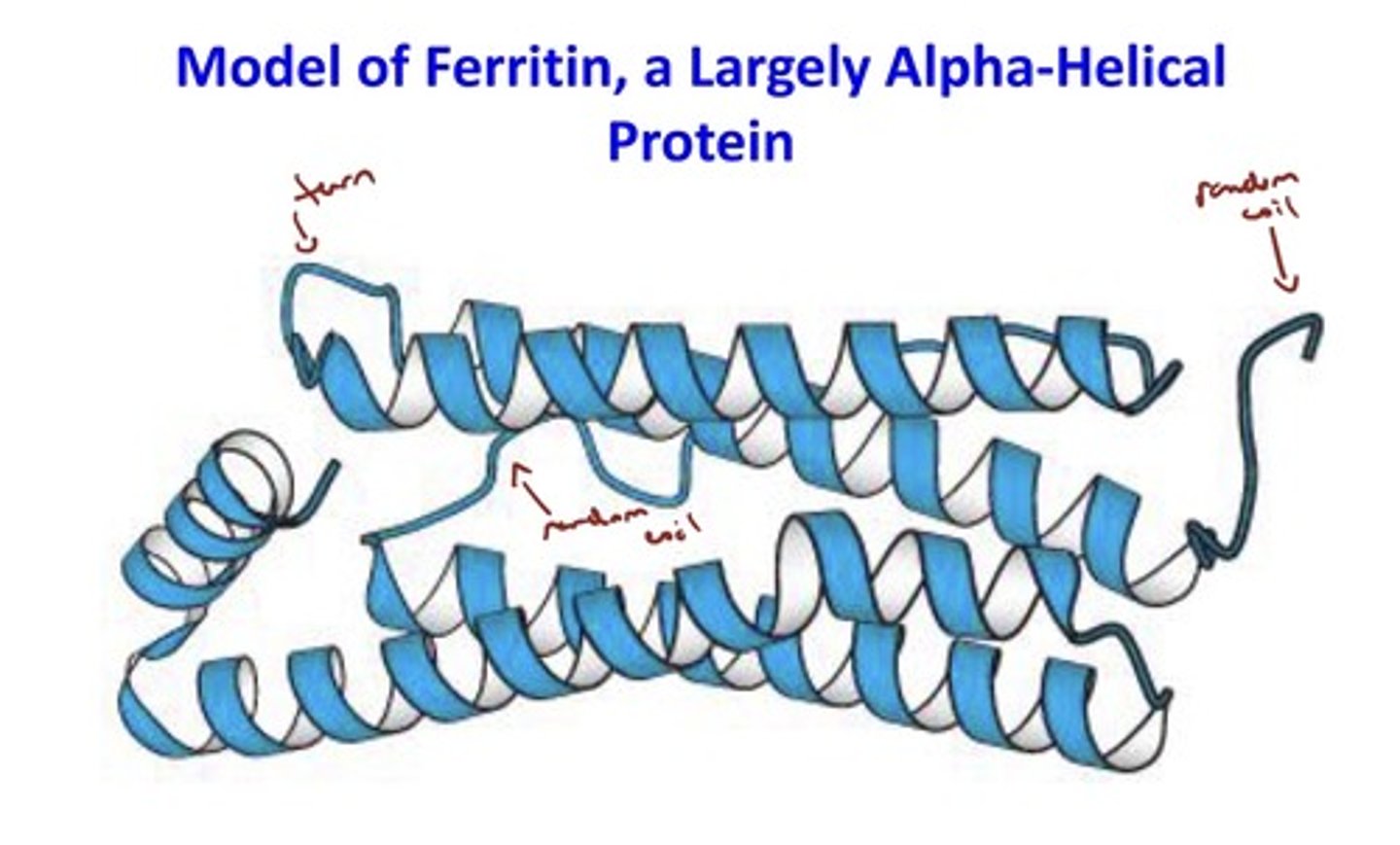
Image of Ferritin
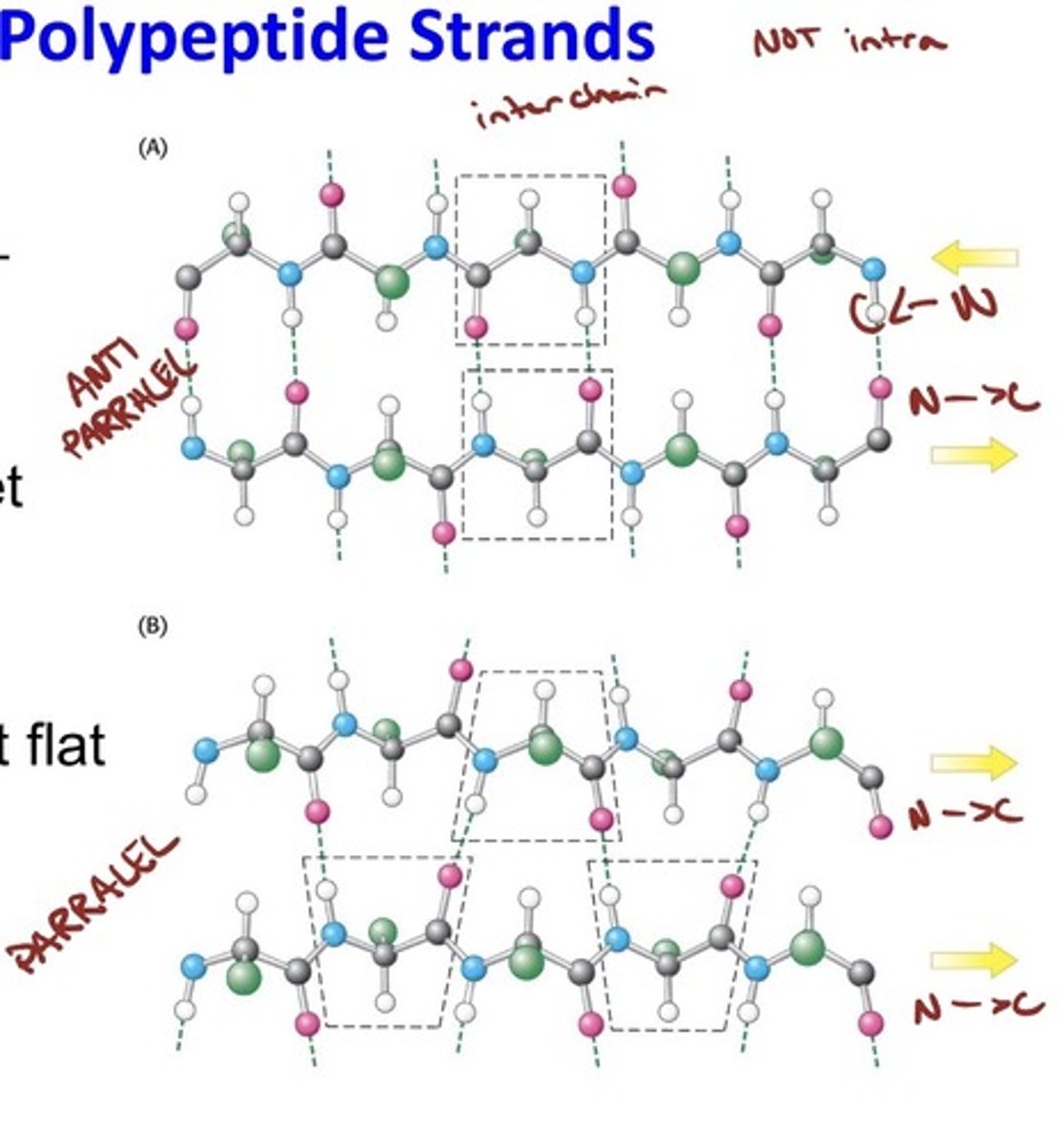
Beta Sheets
- Stabilized by Hydrogen Bonding Between Polypeptide Strands
- The β sheet is another common form of secondary
structure.
• Beta sheets are formed by adjacent β strands.
• In contrast to an α helix, the polypeptide in a β strand is
fully extended.
- Hydrogen bonds link the strands in a β sheet.
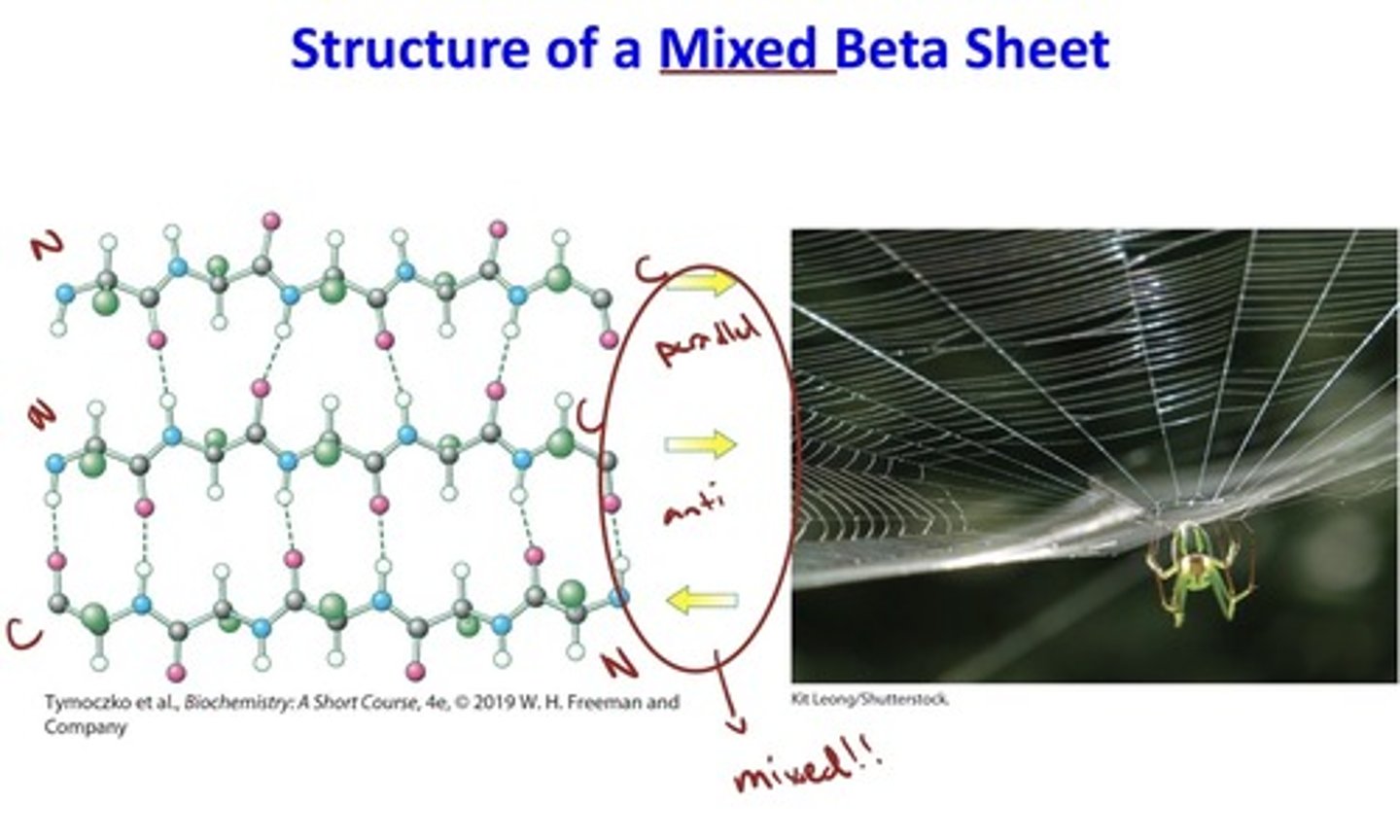
• The strands of a β sheet may be parallel, antiparallel, or mixed.
• β sheets may be almost flat or adopt a twisted conformation.
structure of a mixed beta sheet
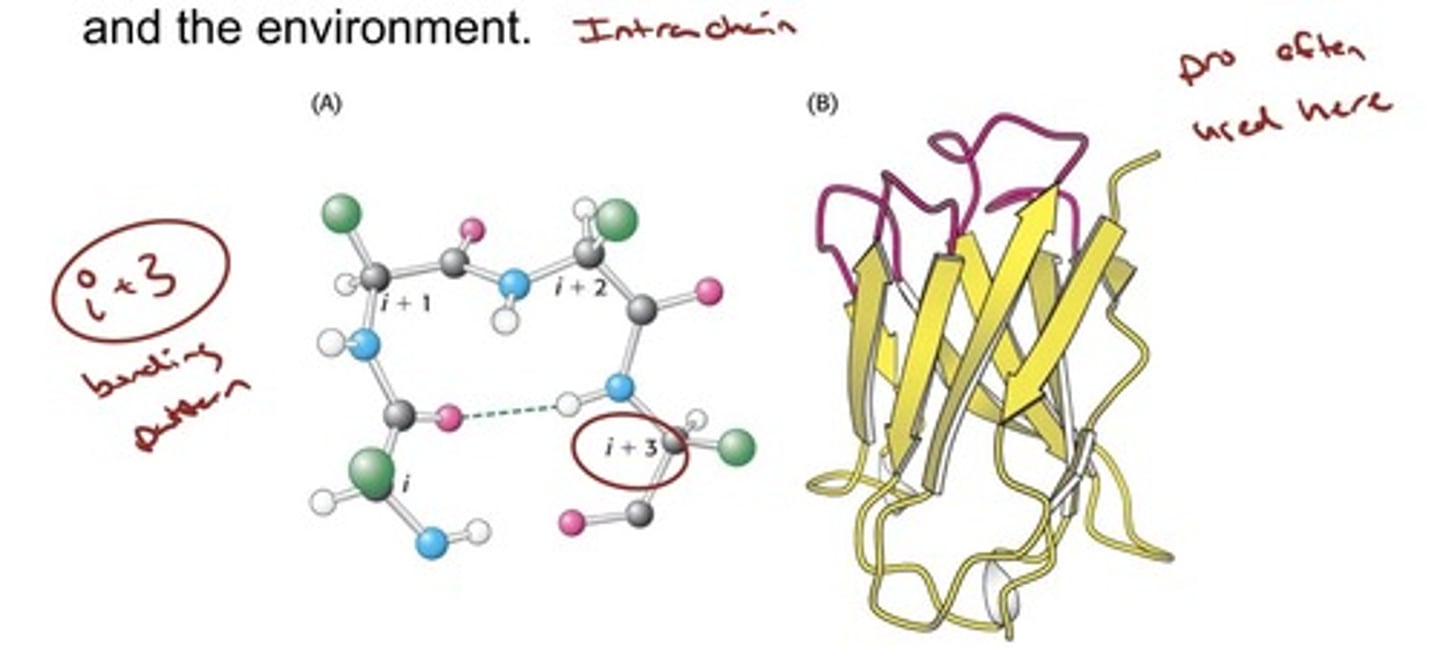
Polypeptide Chains Can Change Direction by
Making Reverse Turns and Loops
- Turns and loops invariably lie on the surfaces of proteins and thus often participate in interactions between other proteins and the environment.
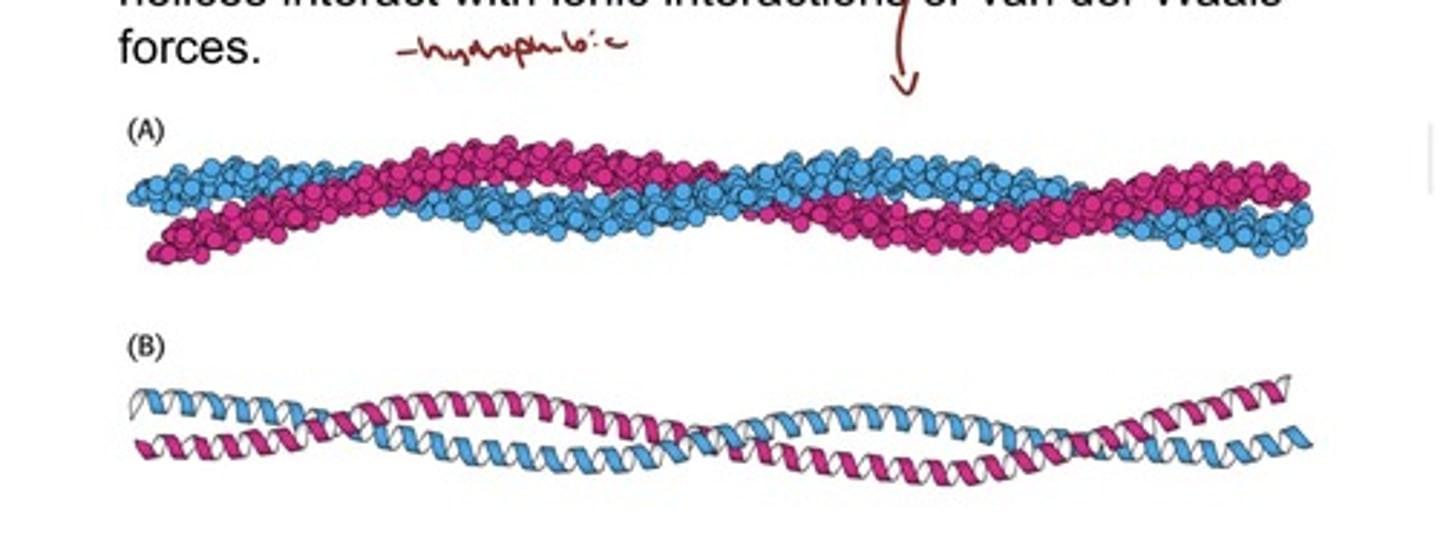
Fibrous Proteins Provide Structural Support for
Cells and Tissues: keratin
- α-Keratin, a structural protein found in wool and hair, is
composed of two right-handed α helices intertwined to
form a left-handed superhelix called a coiled coil
- The helices interact with ionic interactions or van der Waals
forces.
Fibrous Proteins Provide Structural Support for
Cells and Tissues: Collagen
- Collagen is a structural protein that is a component of skin,
bone, tendons, cartilage, and teeth.
• Collagen consists of three intertwined helical polypeptides
chains that form a superhelical cable
- The helical polypeptide chains of collagen are not α helices
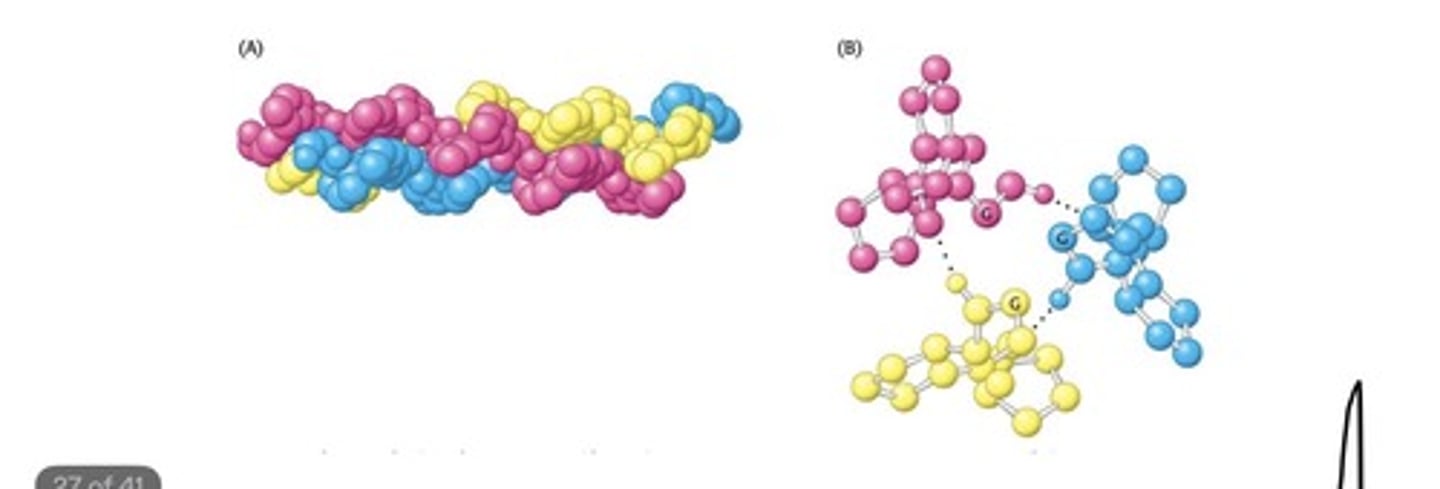
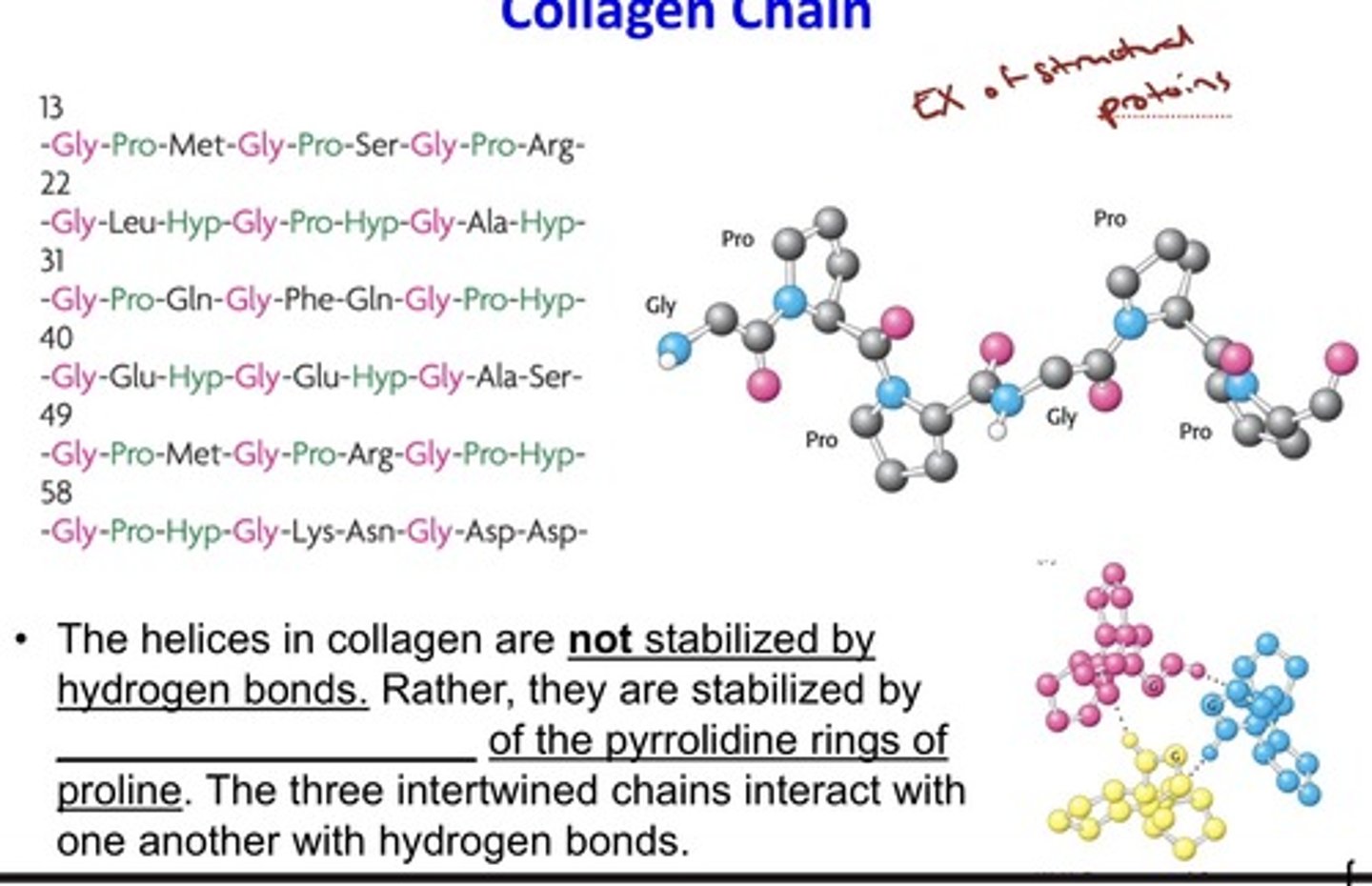
The Amino Acid Sequence of a Part of a
Collagen Chain
- The helices in collagen are not stabilized by
hydrogen bonds. Rather, they are stabilized by
steric repulsion of the pyrrolidine rings of
proline.
- The three intertwined chains interact with
one another with hydrogen bonds
Tertiary Structure
- Water-Soluble Proteins Fold into Compact Structures
- refers to the spatial arrangement of
amino acids that are far apart in the primary structure and
to the pattern of disulfide bond formation
- This level of structure is the result of interactions between
the R groups of the peptide chain.
– Salt bridges (electrostatic)
– Disulfide bonds (cysteine residues)
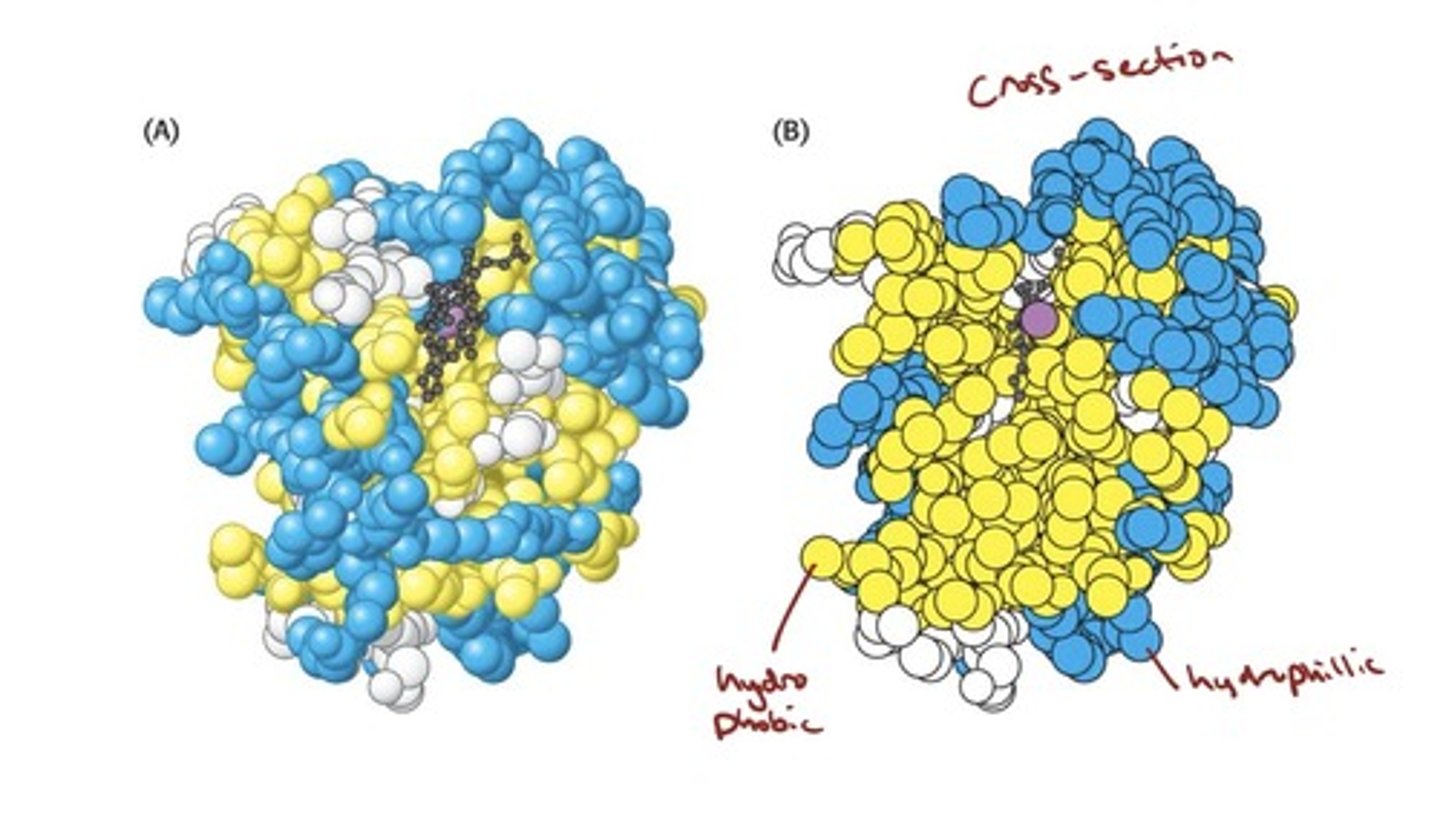
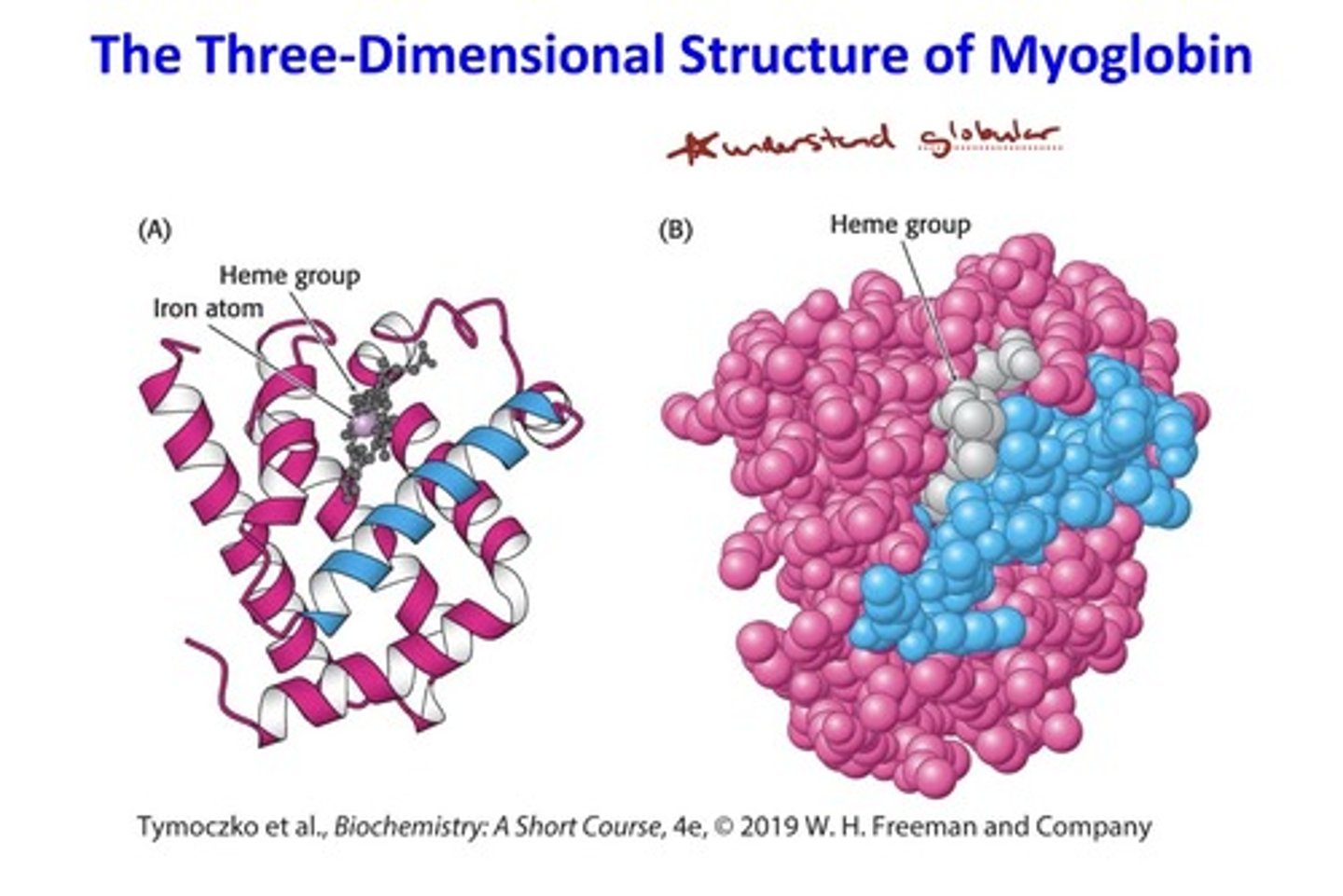
Myoglobin Illustrates the Principles of Tertiary
Structure
- Globular proteins, such as myoglobin, form complicated
three-dimensional structures.
• Globular proteins are very compact; there is little or no
space in the interior of globular proteins.
• The interior of globular proteins consists mainly of
hydrophobic amino acids.
• The exterior of globular proteins consists of charged
and polar amino acids.
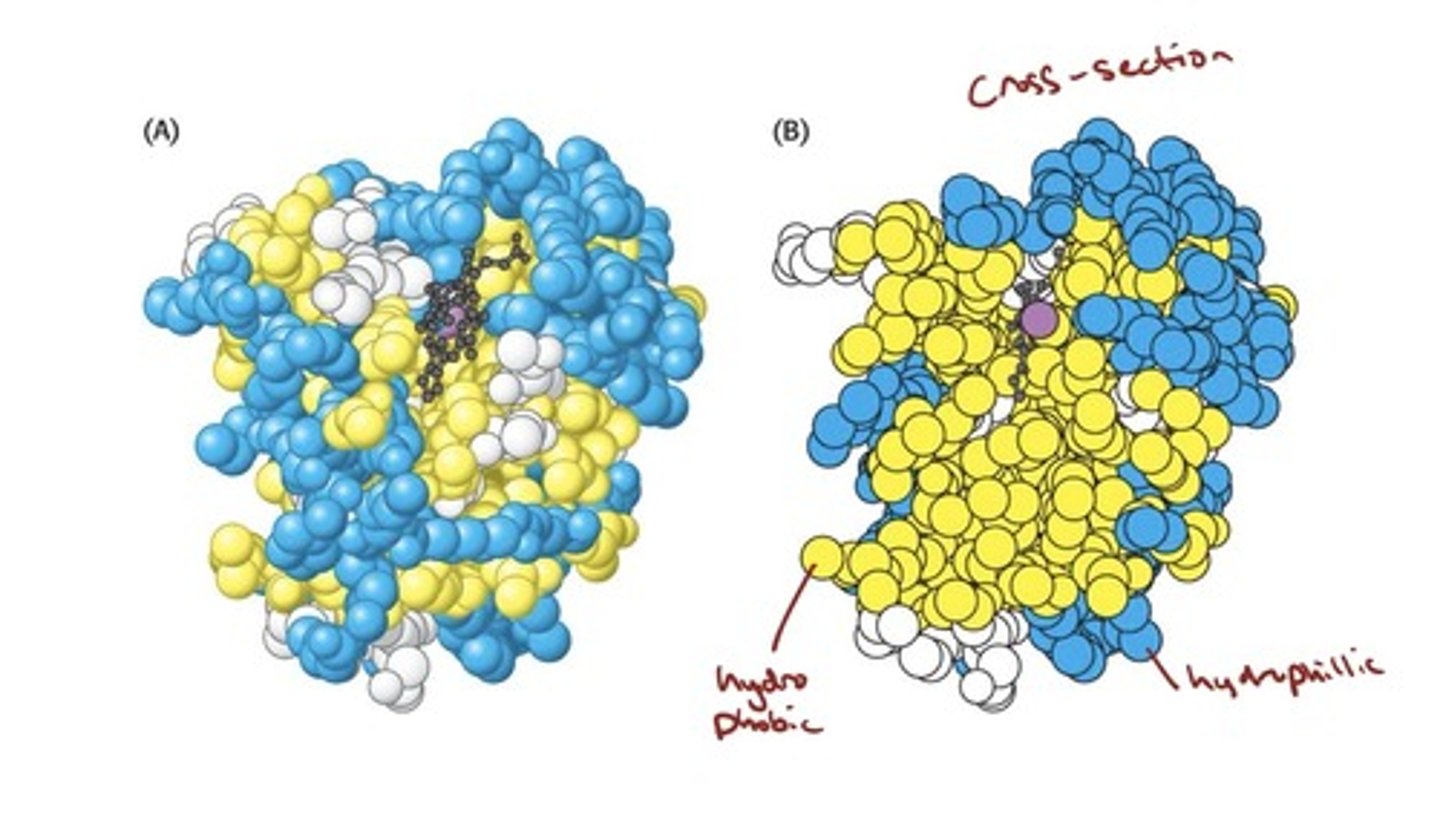
three-dimensional structure of myoglobin
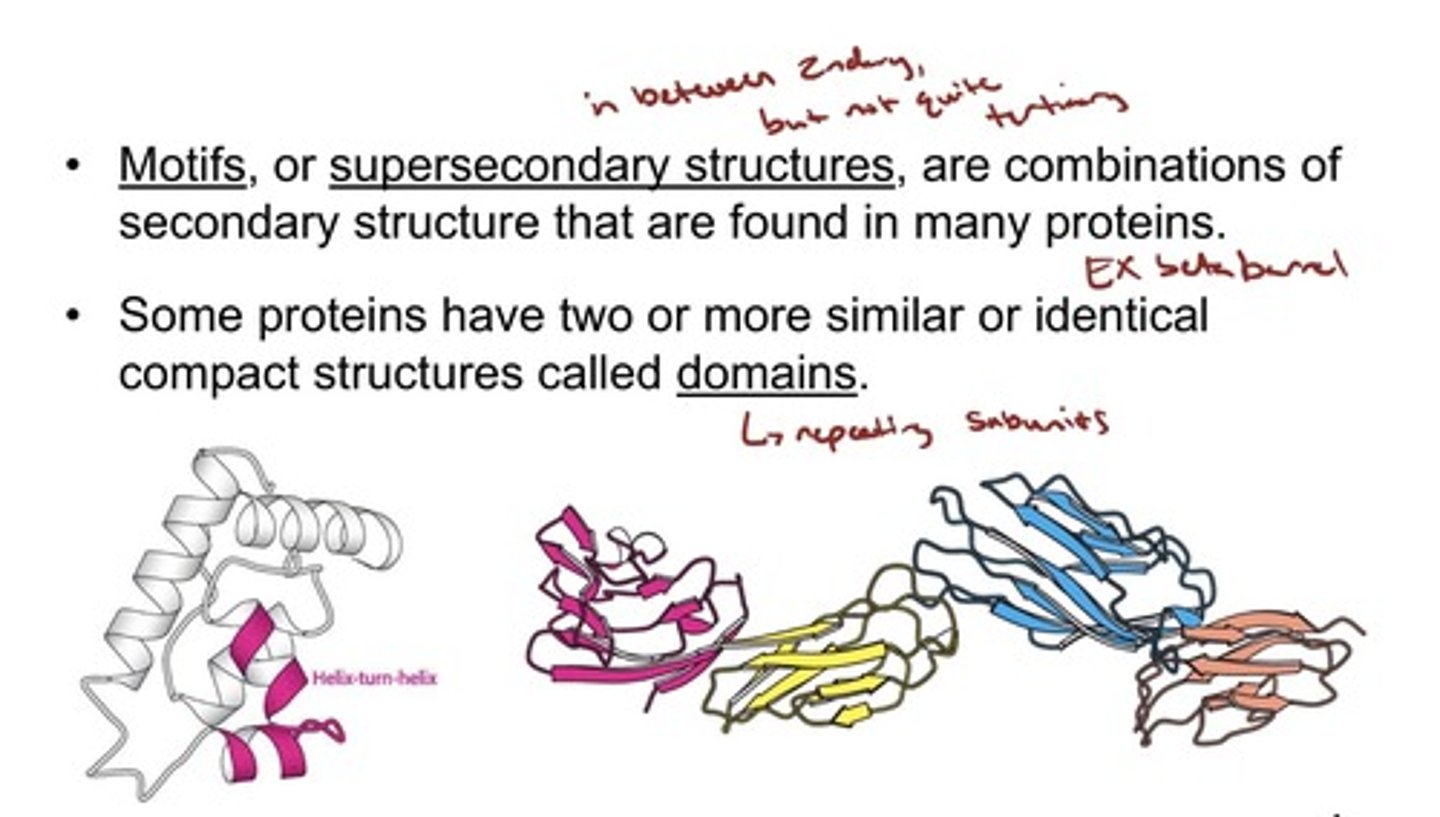
The Tertiary Structure of Many Proteins Can Be
Divided into Structural and Functional Units
- Motifs, or supersecondary structures, are combinations of
secondary structure that are found in many proteins.
• Some proteins have two or more similar or identical
compact structures called domains.
Quaternary Structure
- Multiple Polypeptide Chains Can Assemble into a Single Protein
- Many proteins are composed of multiple polypeptide chains called subunits, or monomers; Such proteins are said to display quaternary structure.
• Quaternary structure can be as simple as two identical polypeptide chains or as complex as dozens of different polypeptide chains.
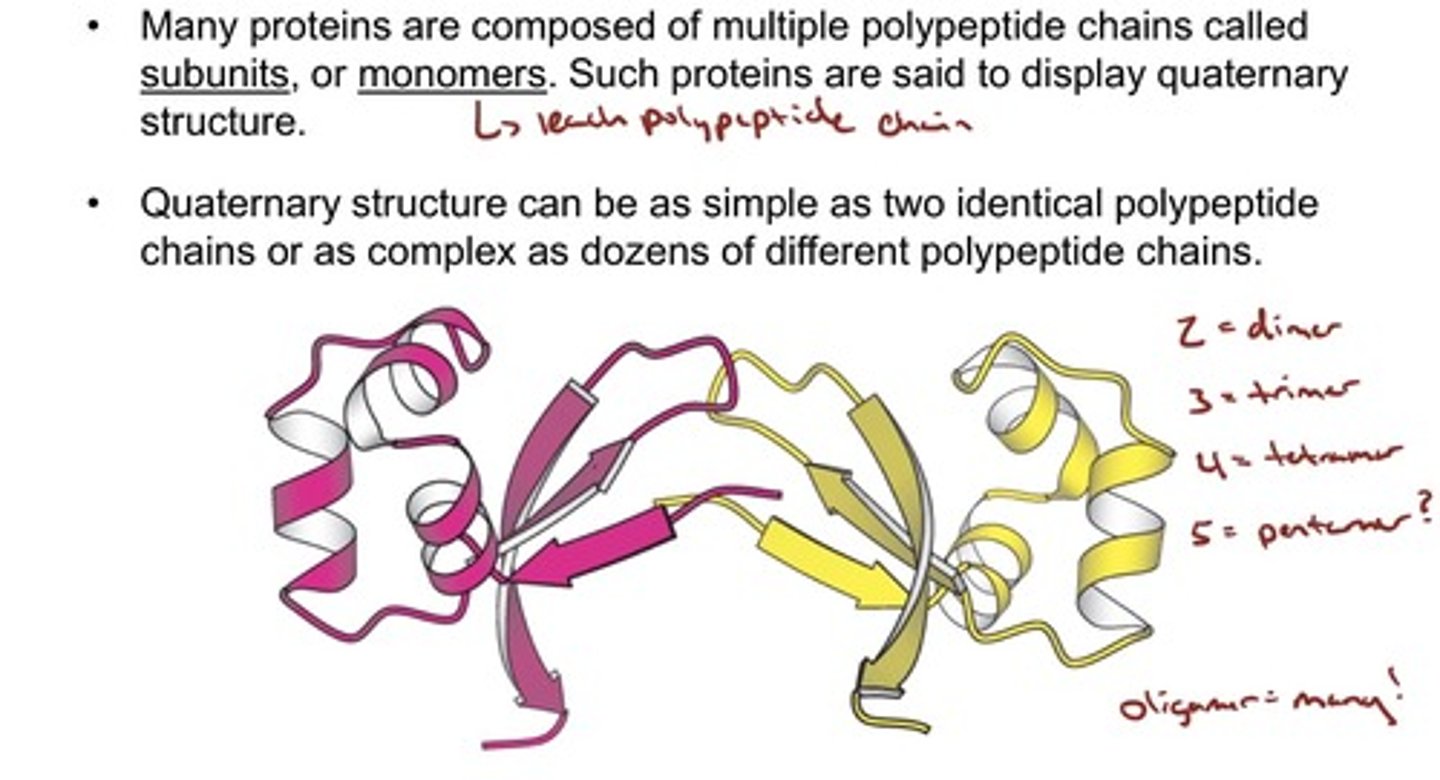
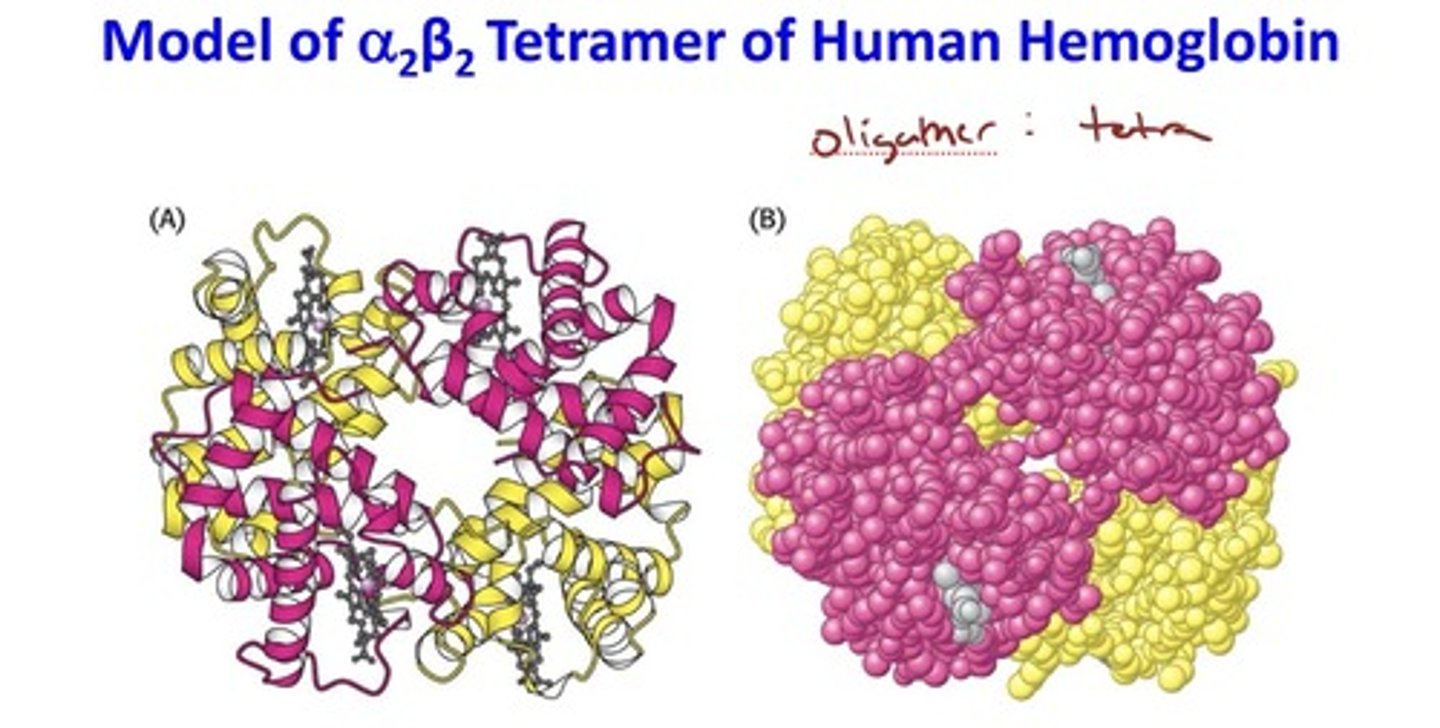
Tetramer of human hemoglobin
The Amino Acid Sequence of a Protein
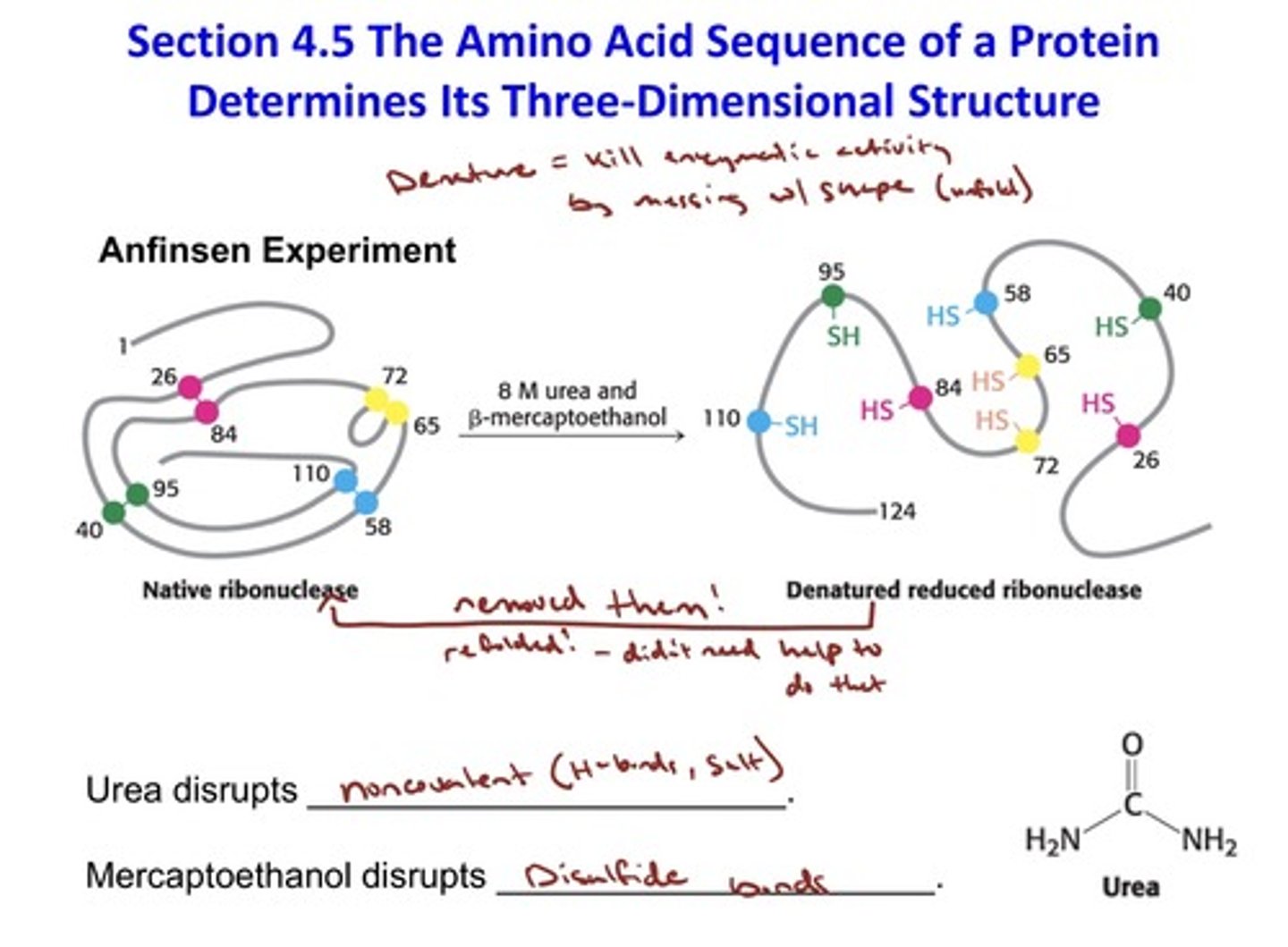
Determines Its Three-Dimensional Structure: Anfinsen Experiment
- urea disrupts noncovalent bonds (between H-bonds and salt bridges)
- Mercaptoethanol disrupts disulfide bridges