Slides L11 Carbonate Rocks
1/55
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
56 Terms
What percentage of Sedimentary Rocks are Limestones and Dolomites?
15% of all SR
How much more abundant are Limestones compared to dolomites?
2-3 times more abundant
Where does most of the CO3 in Carbonate rocks come from?
Mostly comes from the skeletons of organisms
How is Limestone recognized in the field?
Softness (Hardness Test), and Reaction with HCl
Does Dolomite react with HCl?
Dolomite may have a very small to no reaction to HCl
What are the 2 main types of Carbonates?
Limestone and Dolostone
What is Limestone composed of?
Aragonite or Calcite
What is dolostone composed of?
Composed of the mineral dolomite CaMg(CO3)2
What Landscapes are Carbonates found in? Why?
KARST Landscapes. this is because Carbonates are dissolved easily.
What is a KARST Landscape?
A landscape with features such as sink holes, caves, and underground drainage systems. Example: Stalagmite and Stalagcites This is due to their solubility in highly acidic water.
What percentage of all SR are Limestones?
10% of all SR
What is the crystal system of Aragonite and Calcite?
Aragonite - Orthorhombic system
Calcite - Trigonal- rhombohedral system
Rank the following based on stability: Low Mg Calcite, High Mg Calcite, and Aragonite (High to Low stability)
Low Mg Calcite
High Mg Calcite
Aragonite
At what temperature does aragonite change to calcite?
380-470 degrees Celsius
What organisms create Calcite and Aragonite?
Aragonite:
Mollusks
Modern Corals
Calcite:
Brachiopods
Trilobites
Echinoderms
When were “Calcite Seas” common?
Early Paleozoic
Middle to Late Mesozoic
When Earth was warmer than today
When were “Aragonite Seas“ common
Middle Paleozoic
Cenozoic
Today
Occurs during cooler periods
Describe Hydrothermal Altercation
when seafloor spreading is active, seawater passes through hot basaltic crust. Ca-rich minerals are converted into Mg-rich clays through hydrothermal metamorphism. This overall reduces the Mg/Ca ratio of the sea water returning to the oceans causing a favor in Calcite compared to Aragonite.
High Mg/Ca ratio → formation of Mg-rich clays from seawater → Low Mg/Ca ratio = Calcite over Aragonite
What are some factors in the precipitation of Calcium Carbonate?
Increased water movement (organisms and breaking waves) - allows diffusion of CO2 into the atmosphere
Low Pressures - CO2 is released from water
High Temperatures - causes lower concentration of CO2
Purpose is to get rid of CO2
Why would increased pressure decrease CaCO3 ppt?
Because pH decreases with depth
Why does pH decrease with depth?
Cold/ deep water contains more dissolved CO2
Describe what the Carbonate Compensation Depth (CCD) is?
The depth below which CaCO3 cannot accumulate and ppt. This Varies between depths of 3000 to 500m depending on water temperature.
How is the ocean more acidic at greater depths?
There is more CO2 in the water at greater depths, which makes the ocean more acidic.
What forms above the CCD?
Chalk and limestone
How did limestone distribution change overtime?
Shallow marine: Late Proterozoic to modern
Deep Marine - Rare in Ancient and more common in modern
Cave Travertine and spring tufa: both ancient and modern
Lakes- Ancient to modern
Where are most modern limestone deposited today?
mostly deposited in reefs, carbonate sand bodies close to reef and in lagoons.
Where were limestones common in the past?
Were more common due to warmer temperatures and greater area of shallow shelf seas.
What are some Limestone components?
Allochems: equivalent of grains in clastic rocks
Interstitial material (Micrite and cement): equivalent to clay and cement in clastics.
What are the major components to allochems?
Skeletal Grains:
Bivalves and other mollusks
Brachiopods
Echinoderms including Crinoids
Corals and sponges (eg Stromatoporoids)
Calcite Secreting Algae
Non-skeletal Grains:
Ooids- coated grains
Oncoids (Oncoliths) - coated grains
Peloids/ pellets
Intraclasts
What does the character of skeletal grains depend on?
Depends on the environment and the geological period of deposition of a rock. For example: Crinoids were important components of Devonian and Carboniferous Shallow marine settings and significant limestone producers at the time.
What are ooids?
They are inorganically ppt small (<2mm) sedimentary grains that are coated.
What are Ooids composed of?
Commonly composed of Calcium Carbonate, but can also be formed by iron or phosphate based materials.
What is the usual depositional environment for Ooids?
Usually in shallow tropical seas. Example: the Bahamas or Persian Gulf
What is the difference between Pisoids and ooids?
Ooids are < or = 2mm in diameter. Pisoids are >2mm in diameter.
How do ooids form?
Form as concentric layers of material are ppt around a nucleus like a shell fragment or a piece of sand. They grow as they are rolled back and forth over the ocean floor in shallow water conditions.
What are ooids mostly composed of today and anciently?
Most ooids are composed of Aragonite today. Ancient ooids are composed of Calcite forming either by direct ppt during the calcite seas or through the altercation of original Aragonite.
What is an Oncoid (Oncoliths)?
Spherical concentrically layered coated grains formed by photosynthetic cyanobacteria that grow around a nucleus such as a shell fragment.
organically ppt around a nucleus
Cyanobacteria (photosynthetic)
Warm waters within the photic zone
Where do Oncoids form?
In the photic zone (depth to which the sunlight can penetrate to the ocean floor)
How to peloids/pellets form?
Are thought to be fecal pellets (poop) or organisms.
What are intraclasts?
They are irregularly-shaped allochems.
How do intraclasts form?
Form by the erosion of partially lithified sediment which is then re-deposited as irregularly shaped grains (syndepositional).
What rocks are intraclasts common in?
Common in limestones due to the rapid cementation of carbonates
What is Micrite?
“CaCO3 mud“ composed of clay sized crystals.
How does Micrite form?
Forms as calcareous algae skeletons breakdown. The exact mode of formation of ancient Micrite is uncertain.
What are Micrites depositional environments?
Generally indicates quiet (low energy) marine settings where micrite would not be washed away.
What is Sparite?
Calcium Carbonate Cement that fills orignal void spaces
What is Sparite made out of?
Can be either Dolomite or Calcite. Micrite can be recrystallized into sparite.
What are the two different Classification Schemes for Limestones?
Folk Classification Scheme
Dunham Classification Scheme
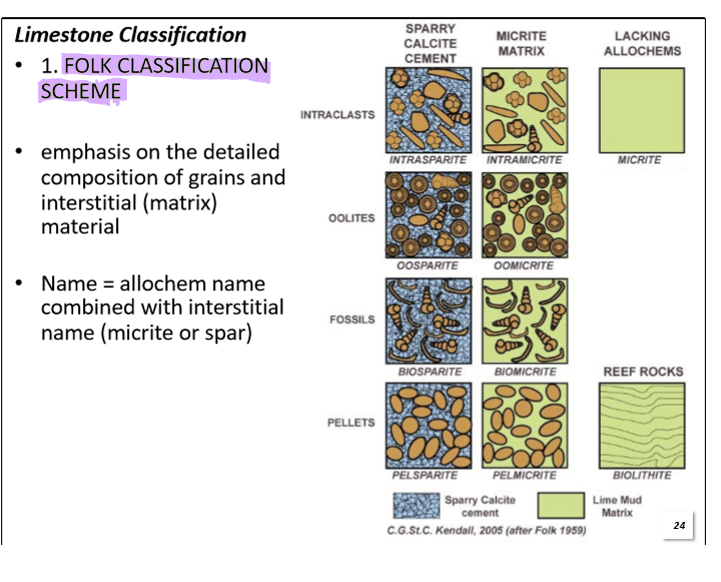
What is the Folk Classification Scheme?
A two part naming system. First name refers to the grains and second refers to the cement or matrix.
Examples:
Oolites + Fossils + spar matrix = Oobiosparite
Pellets + Oolites + fossils + micrite matrix = peloobiomicrite
Note: Most abundant allochem first.
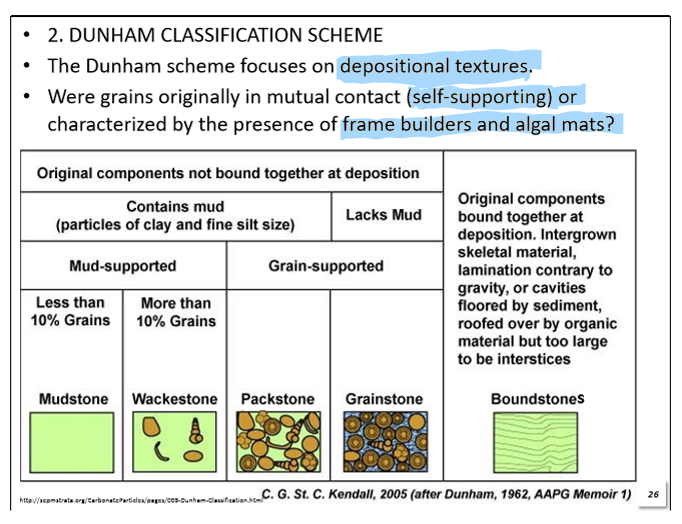
What is the Dunham Classification Scheme?
Classifies using depositional textures. Rocks are divided into four main groups based on proportions of allochems and whether or not they were in contact with each other.
originally in contact = grain supported
not originally in contact = mud supported
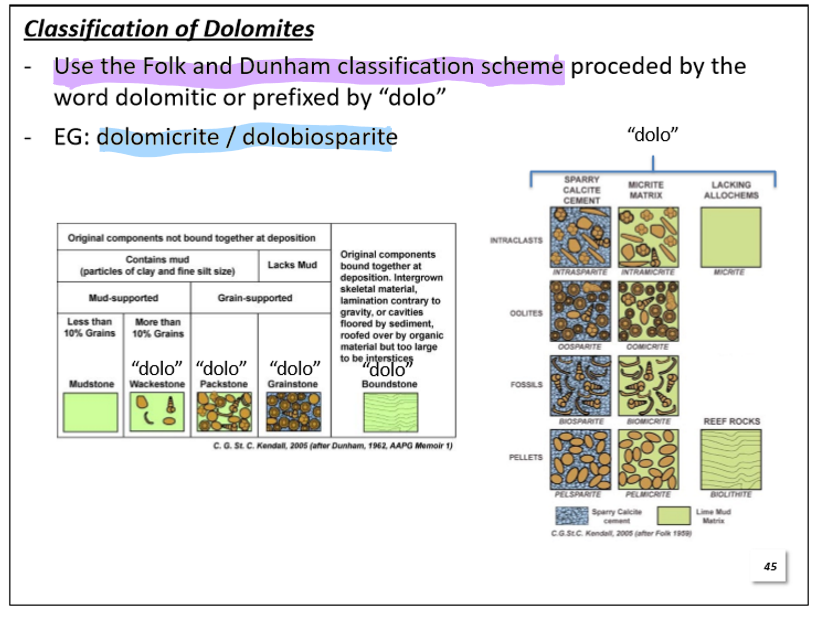
How do we classify Dolomites?
Use the Folk and Dunham classfication scheme prefixed by the word “dolo-“
Example: dolomicrite/dolobiosparite
What is the crystal system of Dolomite?
Trigonal - rhombohedral system
Does dolomite react with HCl?
Does not dissolve rapidly in HCl
What gives dolomite a yellow-brown color or a rose tint?
Fe in crystal structure causes brown to yellow color. Mn Content produces a rose tint.
What are the proportions of dolomite in sediment and their associated names?
Limestone: 0-10% dolomite in rock
Dolomitic Limestone:10-50% dolomite
Calcitic Dolomite: 50-90%
Dolomite: 90-100%