Microtubules
1/70
Earn XP
Description and Tags
This is how you microtubule in Cellular Biology
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
71 Terms
Who are the microtubule monomers?
Dimerized alpha and beta tubulin.
Describe the “charge” separation of the tubulin
Beta tubulin points towards the plus end, alpha-tubulin points towards the minus end.
How many dimers make the circumference of a microtubule?
13
Describe the structure of a tubulin dimer?
In the alpha tubulin dimer, there is a non-exchangeable GTP bound.
The B-Tubulin has an exchangeable GTP binding site.
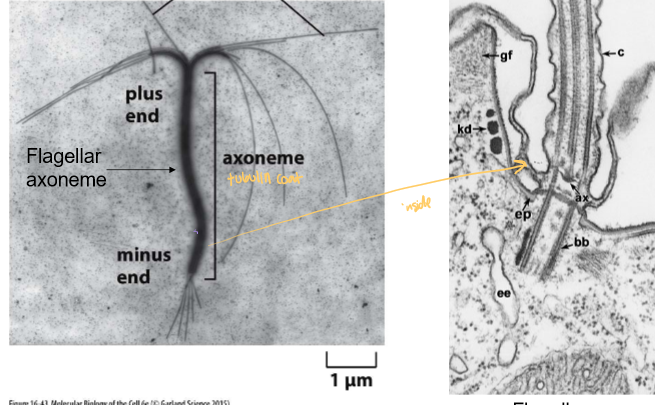
What is an axoneme?
A parallel bundle of microtubules with similar polarity.
The starting point of cilia and flagella
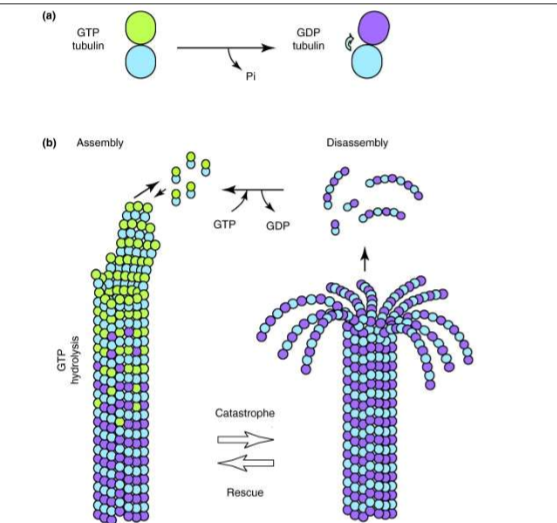
What is a big difference between actin and microtubule growth?
Microtubules don’t treadmill because GDP-bound tubulin cannot polymerize and both ends shrink and grow at the same rate.
Why can’t GDP bound tubulin polymerize?
This is due to its conformational shape. GDP bound tubulin curves and thus prevents the side interactions that keep tubulin monomers connected via lateral (side-by-side) interactions. The KD is very large, while the KA is essentially 0
What is microtubule dynamic instability?
The tendency for tubulin’s stochastic decrease in size when GDP-bound subunits are exposed at the end, leading to catastrophic depolymerization
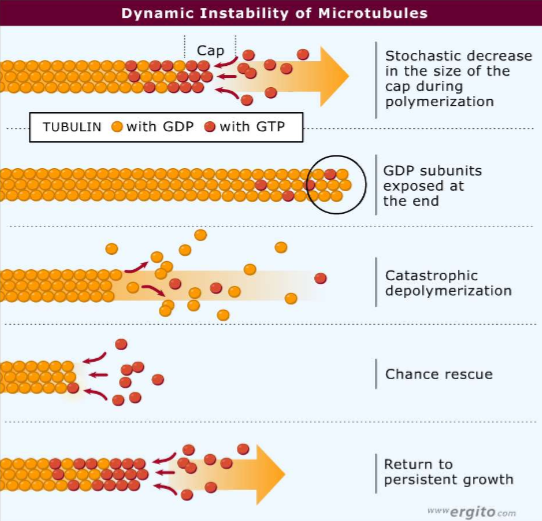
If pure tubulin is depolymerizing catastrophically, what is it’s chance of rescue?
ALMOST ZERO! Other proteins must be there to help!
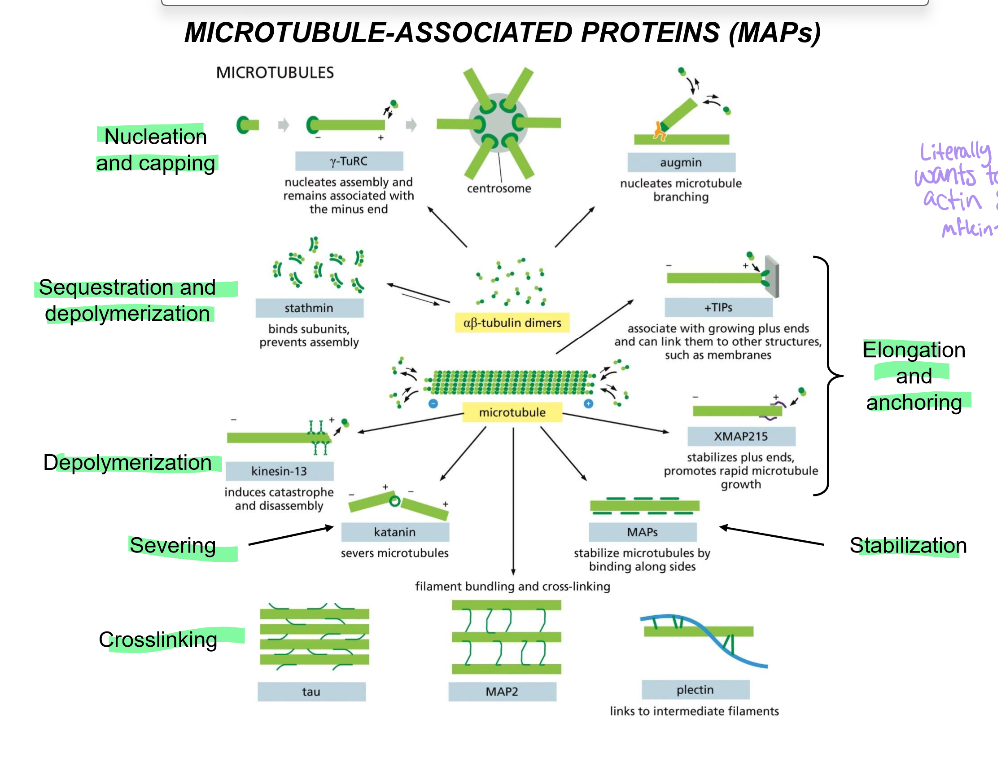
List all the microtubule-associated proteins (MAPs) & their functions
Nucleation and capping:
gamma-TuRC: nucleates and associates with (-) end, cannot cap broken MT strands
centrosome - starting point for all microtubules
camsap - stabilizes newly broken MTs
Augmin - nucleates microtubule branching
Sequestration and depolymerization:
Stathmin: binds microtubule subunits to prevent assembly
Depolymerization:
kinesin-13: induces catastrophes and disassembles
Severing:
Katanin: Severs microtubules in their middles
Crosslinking:
tau, which performs the main crosslinking of the axon
MAP2, which is in charge of the main crosslinking of dendrites
Plectin: links microtubules to intermediate filaments
Stabilization
MAPs stabilize tubules by their sides.
Elongation and anchoring
+TIPs associate with the growing plus ends and links them to other structures, like membranes
XMAP215 - stabilizes plus ends and promotes rapid microtubule growth
List the nucleating microtubule proteins and their functions
gamma-TuRC (nucleates and associates with (-) end)
centrosome - starting point for all microtubules
Augmin - nucleates microtubule branching
camsap - stabilizes newly broken MTs
List the severing, sequestering, and depolymerizing microtubule proteins
Sequestration and depolymerization:
Stathmin: binds microtubule subunits to prevent assembly
Depolymerization:
kinesin-13: induces catastrophes and disassembles
Severing:
Katanin: Severs microtubules in their middles
List the crosslinking, stabilizing, elongation, and anchoring proteins of MTs
Crosslinking:
tau, which performs the main crosslinking of the axon
MAP2, which is in charge of the main crosslinking of dendrites
Plectin: links microtubules to intermediate filaments
Stabilization
MAPs stabilize tubules by their sides.
Elongation and anchoring
+TIPs associate with the growing plus ends and links them to other structures, like membranes
XMAP215 - stabilizes plus ends and promotes rapid microtubule growth
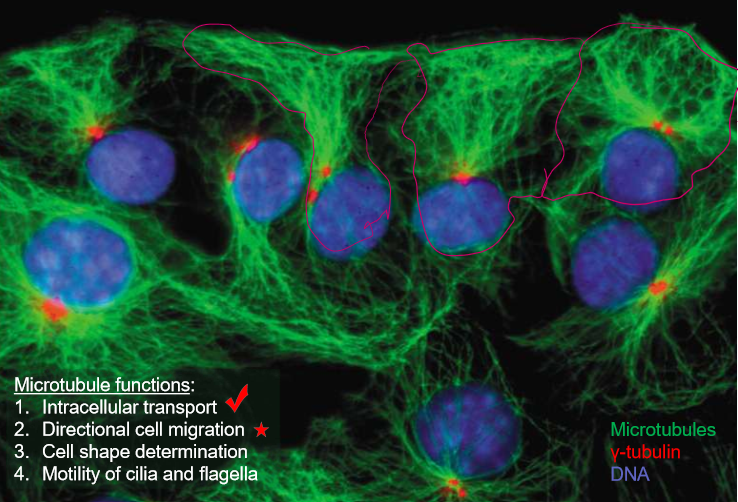
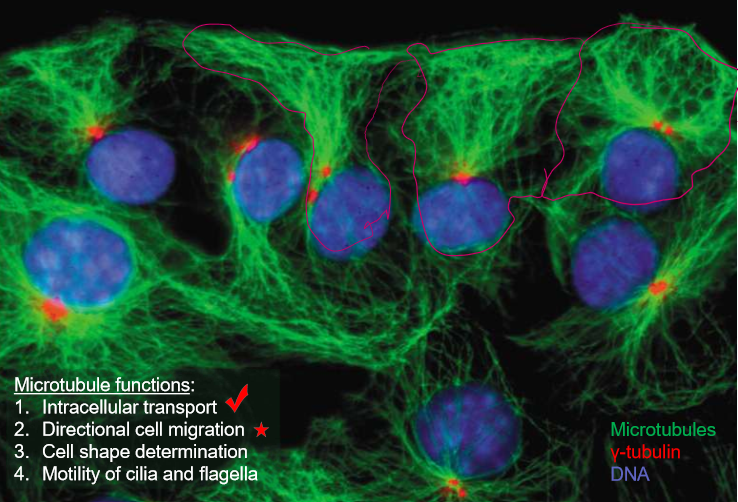
What are the 5 major roles of microtubules?
Intracellular transport
Directional cell migration
Cell shape
Cilia and flagella
Mitosis
In what directions do microtubules orient their poles?
Plus ends go towards the end of the cells, minus ends are towards the inner part of the cell, particularly the centrosomes and the nucleus.
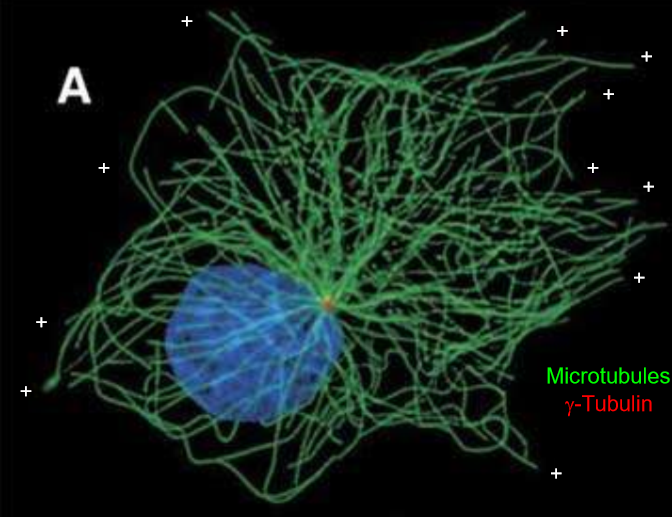
Which parts of the cell have the highest density of microtubules.
The front of the cell has the highest microtubule density, while the back of the cell has the least. This allows the Rho-Rac inhibition to make sense.
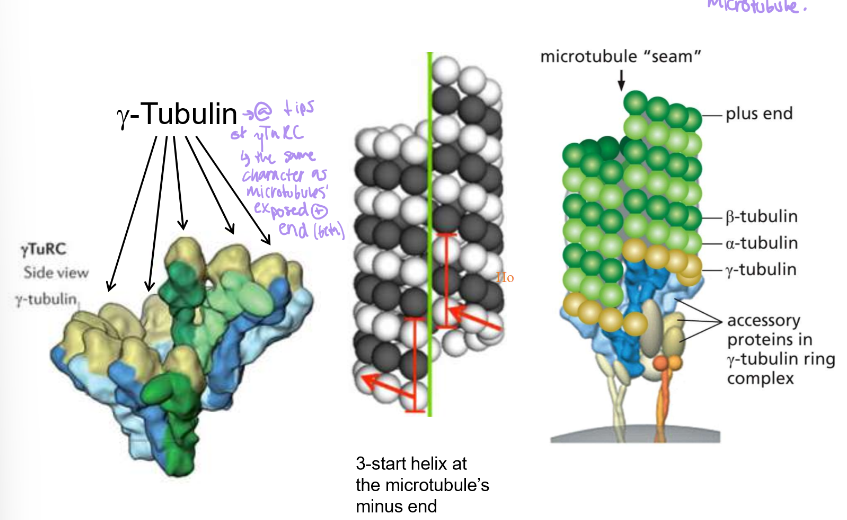
Where do microtubules come from/originate?
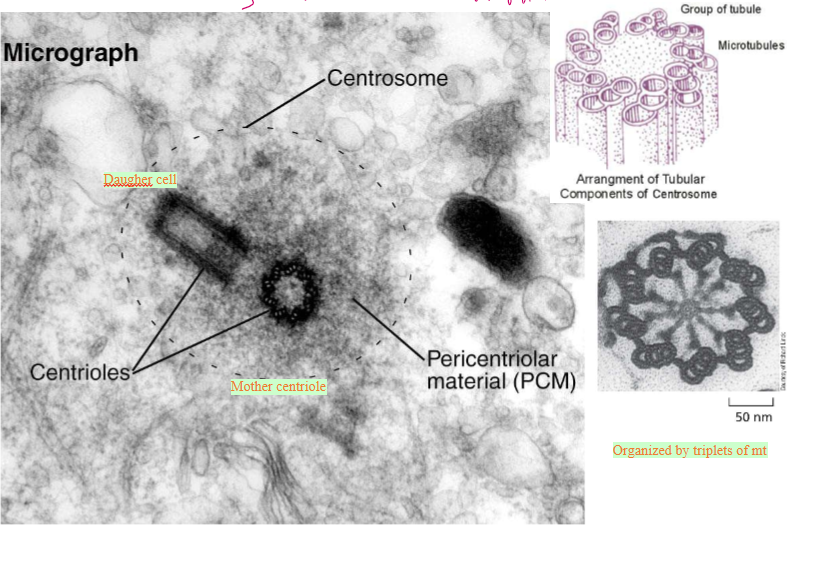
The pericentriolar material (PCM) around the centrioles. This is essentially the centrosome
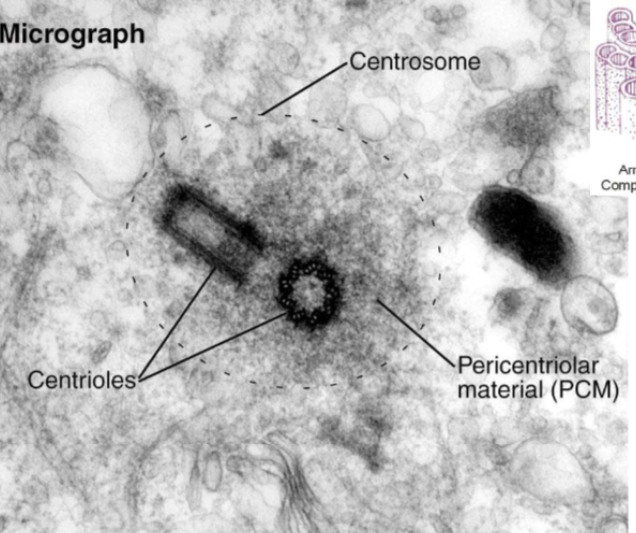
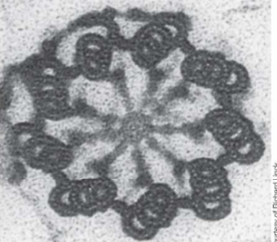
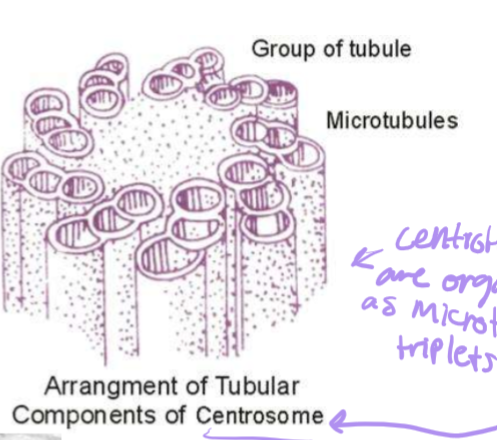
How are centrosioles organized?
Triplets of 8 tubule groups. The alpha-microtubule has a whole wall, the beta-microtubule is in the middle, and the gamma-microtubule is at the end.
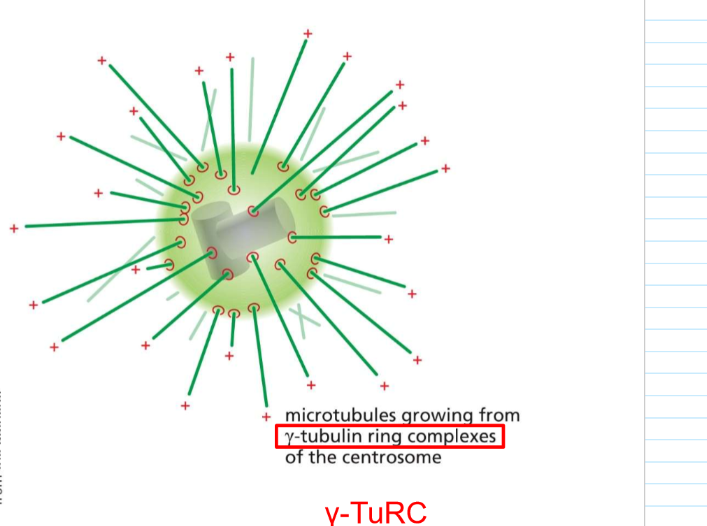
Where particularly do microtubules grow from?
often, minus ends grow from gamma-tubulin ring complexes from the centrosome: Gamma-TuRC
Nucleation is a rate-limiting step. How is this seen in microtubulin?
Favorable growth only happens once two rings have formed, essentially forming the seam that has three tubulin monomers stacked
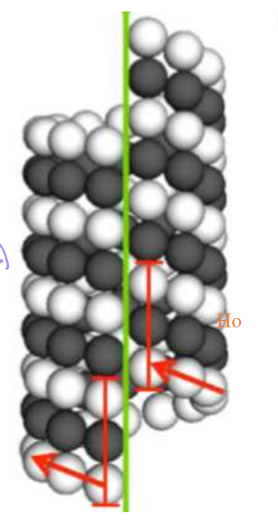
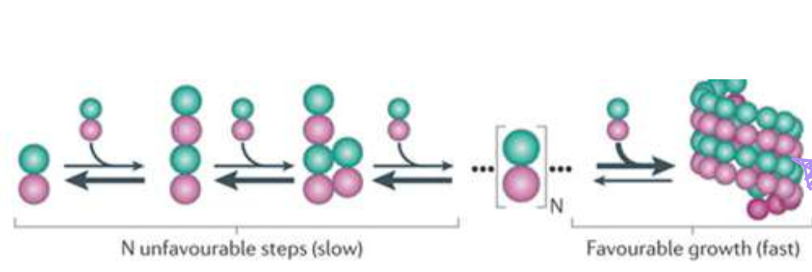
How does gamma-TuRC promote nucleation?
They essentially serve as a template. Their shape an exposed plus end that tubulin would nucleate and stack on
What is gamma-tubulin analogous to in actin?
The Arp2/3 complexes.
What is augmin’s role?
It nucleates microtubule branching, not to generate pushing force but to generate more branching pathways. They’re very important in the axons of neurons.
Where is gamma-TuRC generally localized?
In the centrosome/ PCM (pericentriolar material). However, it’s not present in cells WITHOUT radial organization.
Tips of neurons have WHAT nucleating/capping proteins and where?
In golgi outposts, there are gamma-TuRC
Describe the activity of centrosomes in young vs mature neurons
Young: high centrosomal gamma-TuRC activuty
Mature: inactive centrosomal gamma-TuRC activity
Describe the microtubule polarity of axons vs dendrites?
axons = positive ends push out for clear signaling
dendritintes = observed mixed polarity
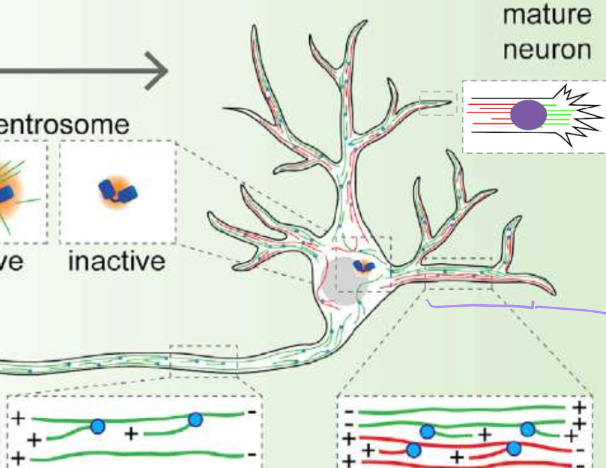
How are microtubules nucleated in axons
From pre-existing microtubules that are capped at the minus end by augmin. This staggers the growing lengths to fill the long stretches of axons
How are microtubules nucleated in dendrites
From y-TuRCs around endosomes (golgi count as endosomeshere)
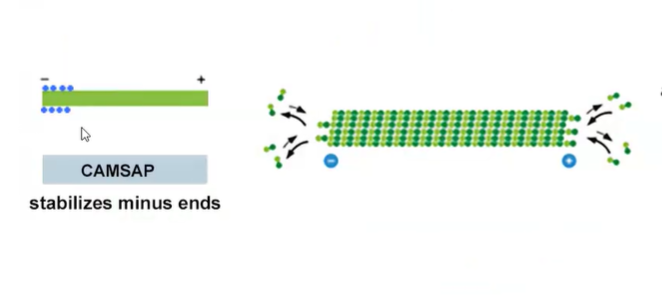
How does CAMSAP work?
By stabilizing broken minus ends in microtubules, CAMSAP functions in parallel with gamma-TuRC
this is bc gamma-TuRC cannot stabilize broken microtubules
What are +TIPs and which face of the tubulin dimer does it attach to.
they’re a large family of + end stabilizers
attaches to beta-tubulin to link to other structures like membranes.
Also stabilizes tubulin dimers to prevent catastrophes, & promotes microtubule rescues
EB (end binding) +TIP proteins recognize GTP cap/GTP bound Beta-Tubulin will recruit other +TIPs → great for rescue
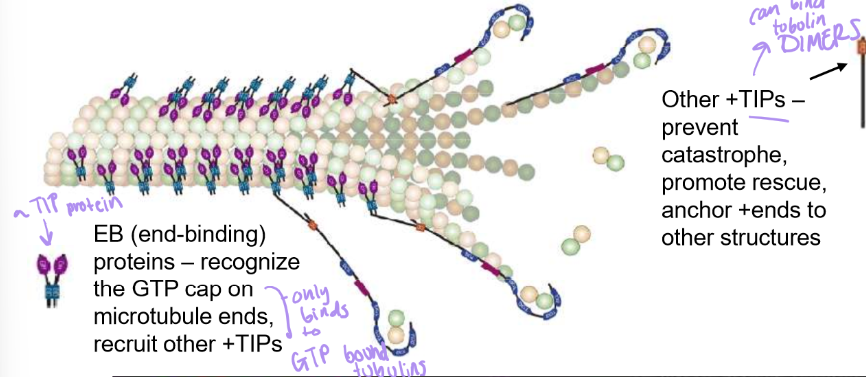
what is XMAP215 and how does it work?
Like +TIPs , they also stabilize plus ends, but they also promote rapid microtubule growth by recruiting tubulin dimers to + ends, almost analogous to VASP.
How are non-radial microtubules organized in epithelial cells?
The basal surface, which is in contact with other surface tissues, has plus-end orientation
The apical surface that faces the lumen hosts the minus end
This set up helps collect resources from the lumen and bring them DOWN into the tissue
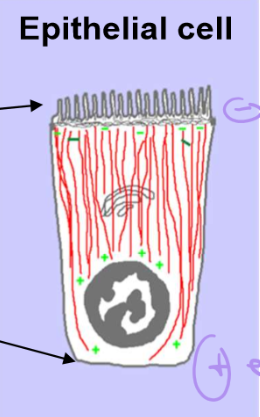
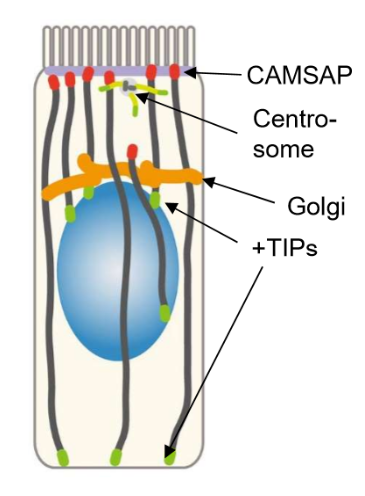
Describe the orientation of capping and nucleators in epithelial cells
Centrosomes and CAMSAP are concentrated on the apical surface
Microtubules will then be cut off from centrosomes by katanin proteins, and then will be stabilized by CAMSAP
CAMSAP and anchor proteins also stabilize and anchor these ends to the apical surface
+TIPs are focused on the basal surface and the nucleus.
These are used to push up, recruit, and elongate microtubules towards the basal surface.
Golgi is by the nucleus
Describe the common arrays of microtubules
Radial: from center to outer-edge: to plus. Radiates from golgis and centrosomes.
Ex) Fibroblast organization
Linear: from cell edge to cell edge: plus or minus down like a line
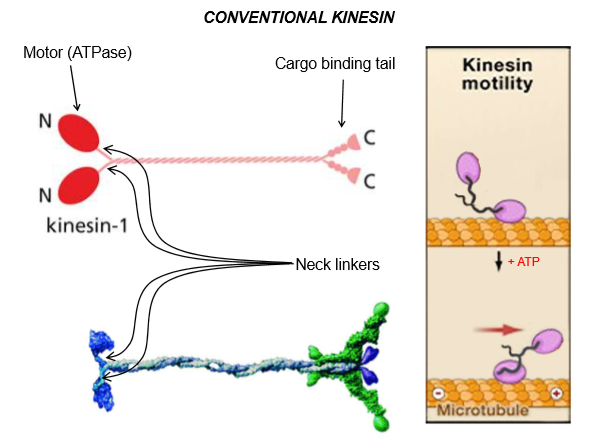
Describe kinesin structure
Motor ATPase head at the n-terminus
Light-chains bind to the cargo-binding tail at c-terminus
Overall, it’s a dimer built like myosin. The atp-bound head stays attached to the microtubule
These neck linkers are flexible
ATP binding will make the neck flip and move forward along the motor
Describe the kinesin kinetic cycle
The ADP-bound head looks for a binding site on the MT until ATP is bound to it
When ATP binds, the binding affinity changes and the head moves up and BINDS STRONGLY to the microtubule
The binding will trigger the power stroke, which swings the second head forward
Then that second head have it’s ADP exchanged with ATP to help it bind to the microtubule
ATP hydrolysis in head 1 is triggered by ADP→ ATP exchange and microtubule binding in head 2..
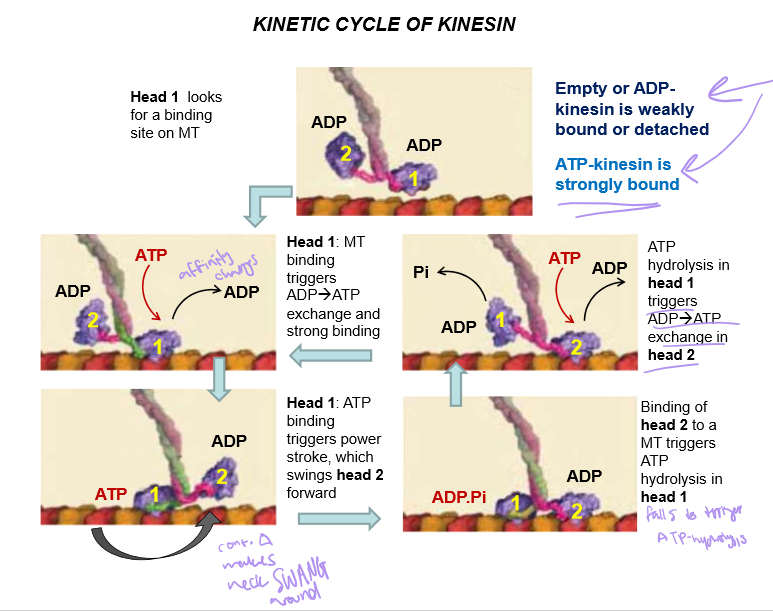
How is empty/ADP bound kinesin’s binding affinity to MT?
WEAK
In what direction does kinesin move?
Towards the plus end
In what direction does Dynein move?
Towards the minus end
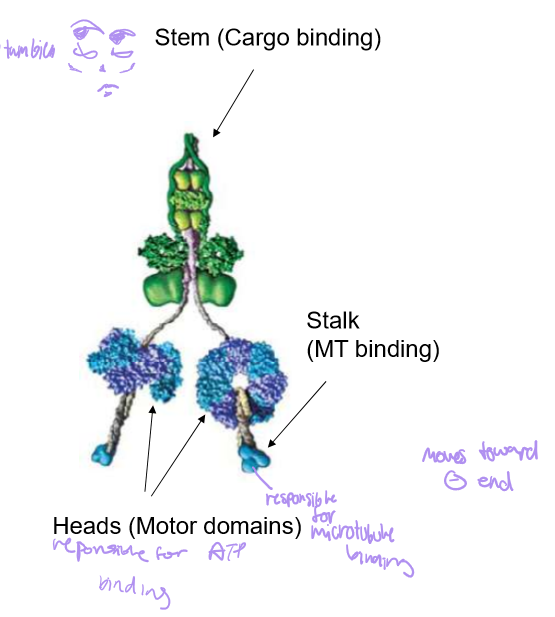
Describe Dynein’s structure. Also discuss the difference between cytoplasmic dynein vs ciliary/flagellar dynein.
Both dyneins are essentially the same, but ciliary dynein has 3 motor heads (same with some axonemal dyneins) instead of cytoplasmal’s 2
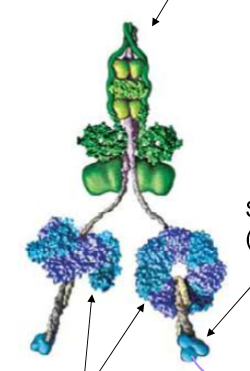
Describe Dynein’s structure
It’s stalk is for MT binding, with circular motor heads (responsible for ATP binding) and a stem for cargo binding
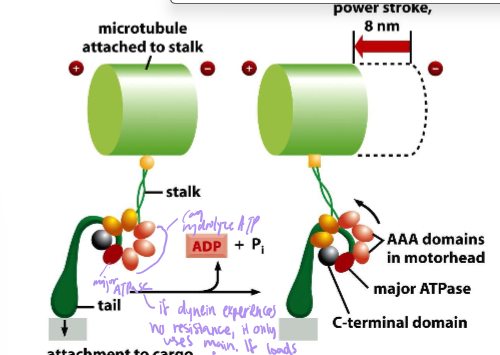
Describe the power stroke of Dynein
length: 8nm
AAA motor head domains seven subunits. Only one is the major ATPase, but if dynein has resistance, it will use all subunits
Its motion is akin to a hella fast drunken sailor: stumbling forwards, backwards, and side-to-side
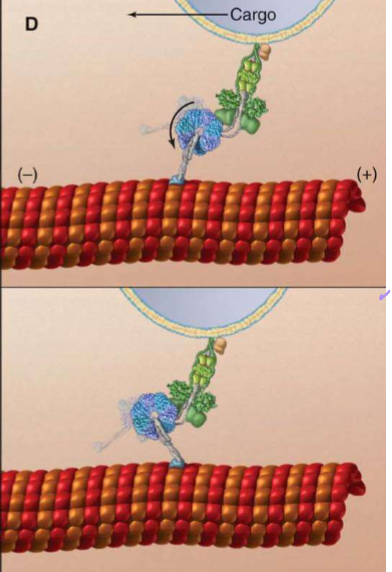
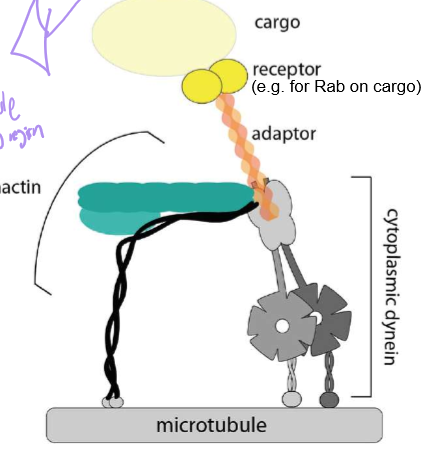
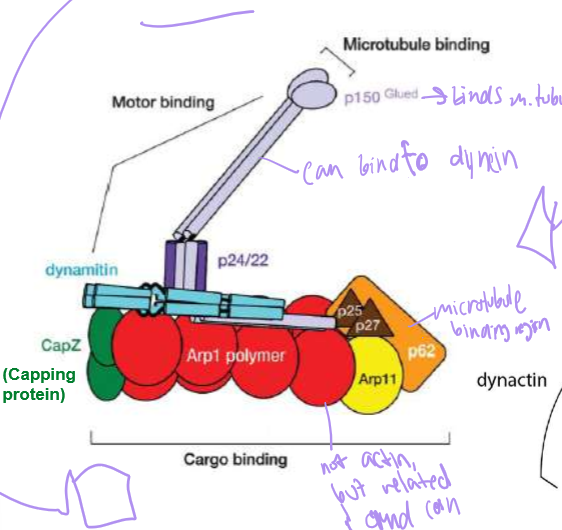
Who is Dynactin?
Dynein’s cofactor: it has another end for microtubule binding, an ARP1 (that’s surprisingly not actin) polymer capped with Cap Z, and topped with dynamatin. They help stabilize dynein’s steps and cargo
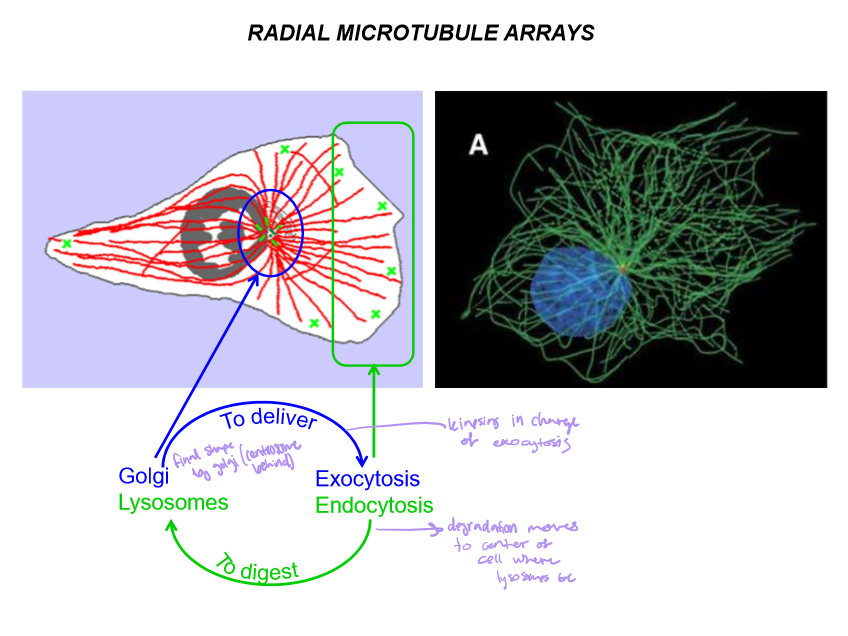
Describe the directionality and the girls in charge of exo and endocytosis
Kinesins - In charge of exocytosis from Golgi (in center of cell) to extracellular space
Dynein in charge of endocytosis from endosome to lysosomes (in center of cell)
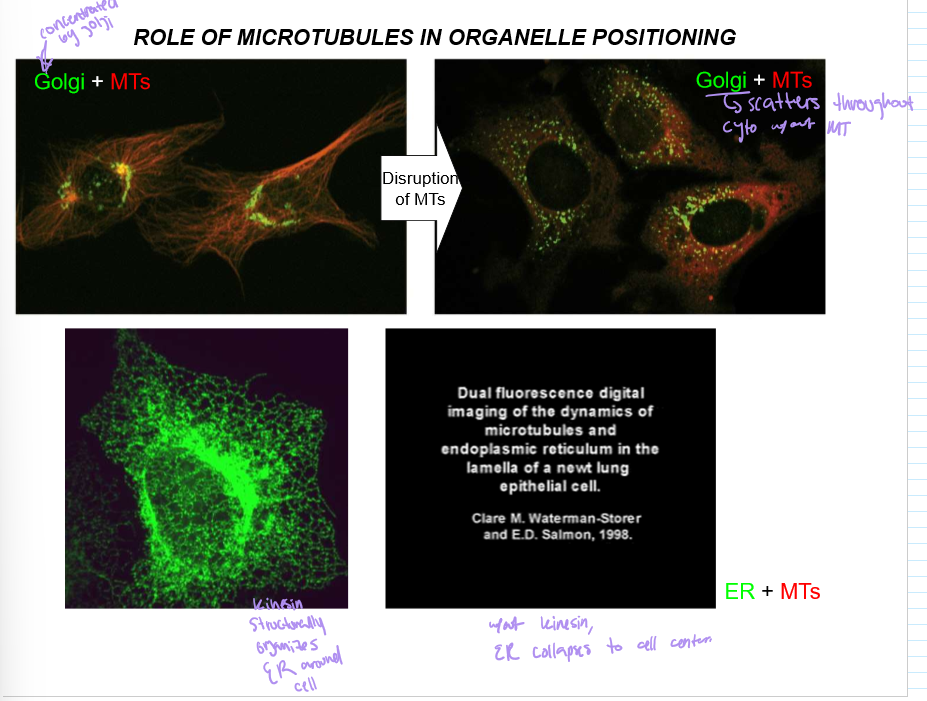
What is the role of microtubules in organelle positioning?
Microtubules organize the Golgi to be around the nucleus, and Kinesin will concentrate the ER around the nucleus too
Without it, the ER collapses around the cell, as does the golgi
What do most degenerative disease in neurons stem from?
Issues in extracellular transport due to issues with vesicle movement along the microtubules, which make up like, 90% of an axon
How are microtubules and their motor proteins organized in axonal transport?
Kinesin move mitochondria and vesicles antrograde: towards the axon terminal
Dynein move damaged retrograde mitochonria and multivesicular bodies that must be recycled retrograde: in towards the dendrite body
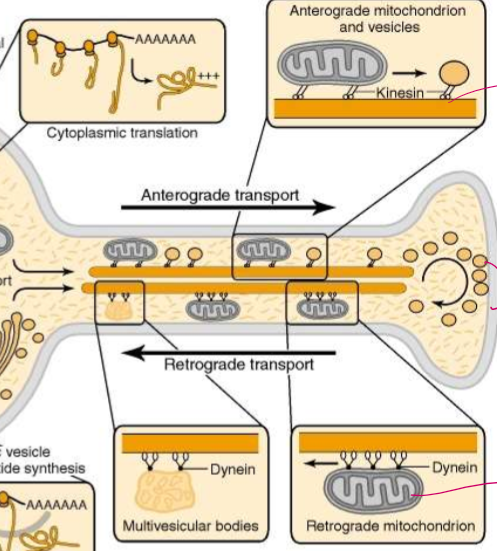
List the steps of mitochondria microtubule motors in axonal transport
1) Mitochondrial polypeptides are synthesized and imported into the mitocondria.
2) They’re carried by kinesin for anterograde transport
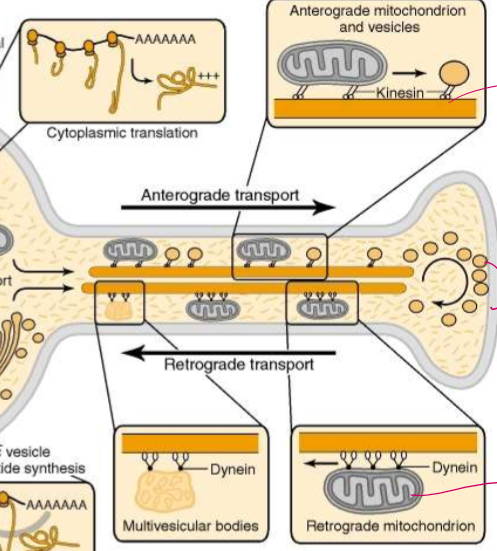
Do vesicles have only kinesin or dynein? Why or why not?
NO! They have both for bidirectional movement! This allows vesicles to move around obstacles. Each kinesin is localized to 1 microtubule, but dynein can move backwards or to different tubules as needed.
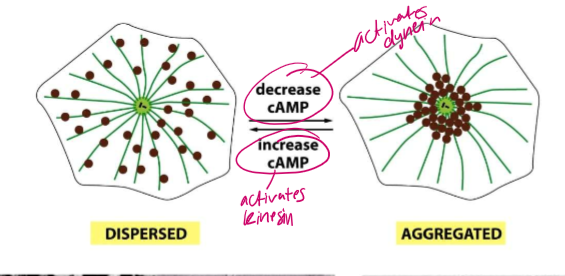
What nucleotide energy signal can activate kinesin? How can its concentration affect dynein?
cAMP!
An increase in cAMP can activate kinesin.
A decrease in cAMP can activate dynein.
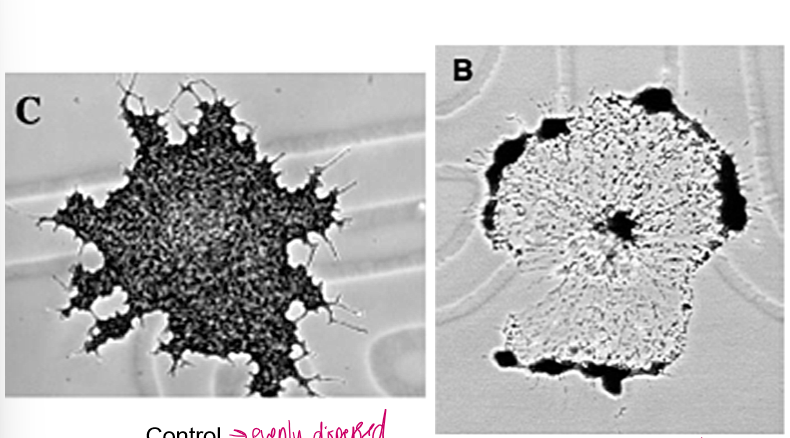
Why is kinesin able to evenly distribute melanin granules in fish cells?
Actin tubules can distract and make kinesin lose its way, but these melanin granules ALSO have myosin V binding domains!
Thus, they can travel along the webs of actin and evenly disperse. However, these roads are finicky, treadmill, and whatnot!
Ultimately, they never get to the edge of the cell and the melanin gets evenly distributed.
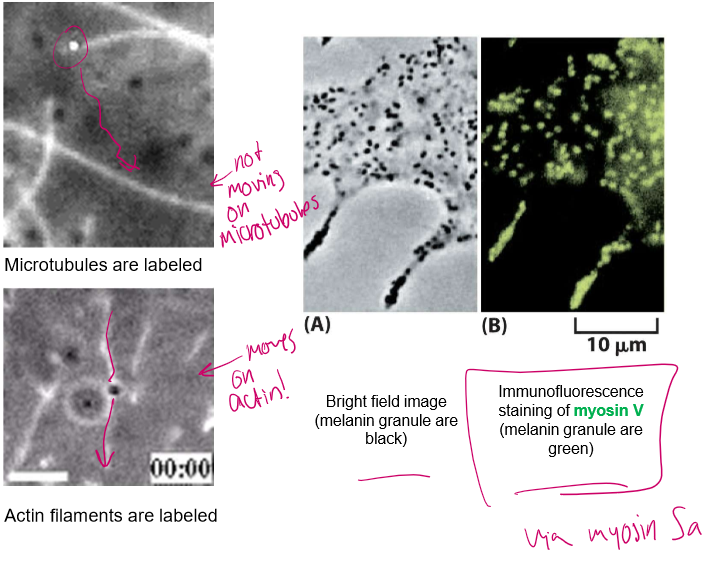
So who all is really on these moving cargos?
Myosin → for shorter, twisting actin roads that microtubules can’t reach
Kinesin & Dynein → directional motors for long, straight cell highways
Gist: How do microtubules help in cell migration?
They push cells forward through the leading edge, and upon contact with other cells, stop moving!
How do cancer cells defy the microtubule wishes in directional cell migration?
By refusing to acknowledge other cell contact, cancer cells begin to climb onto of other cells before growing and replicating
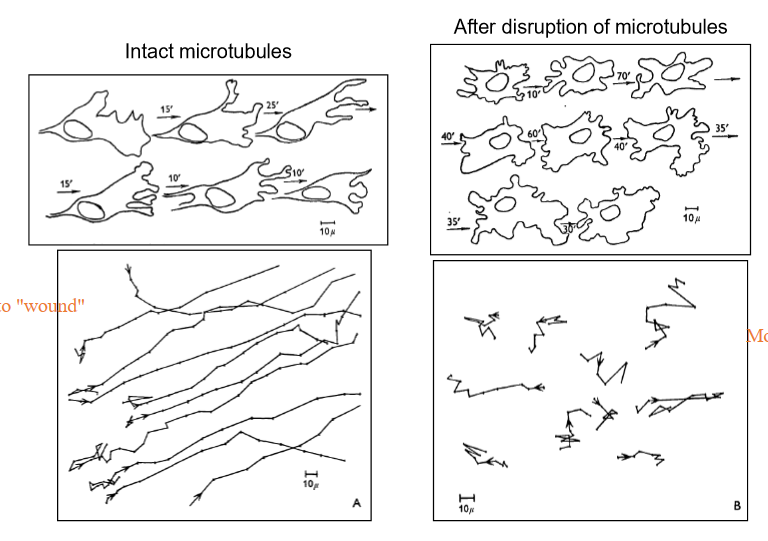
Describe what happens when you disrupt microtubules in terms of cell directional migration?
Cells, which usually move in relatively uniform paths towards their target location, will move erratically not towards their location (usually). It also depolarizes the cell; cells cann'ot tell their rear from their front!
Details: How do microtubules play a part in cell directional migration
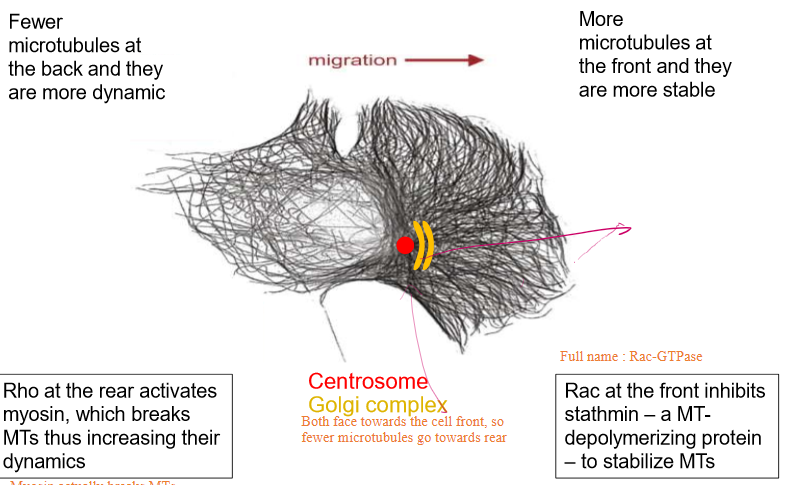
Fewer microtubules turn towards the back of the cell to signify the rear
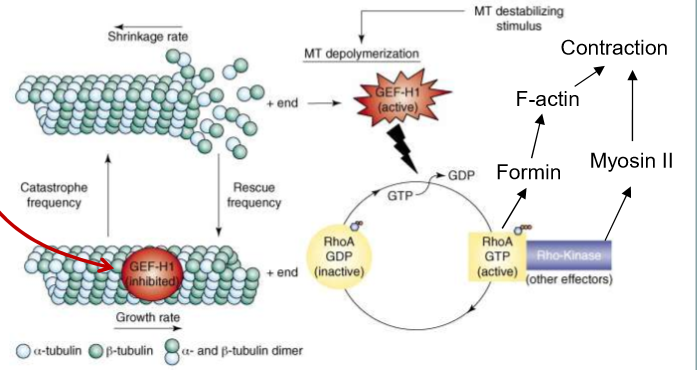
These are more dynamic than the ones at the front and are broken down furthermore by Rho-GTPase
More microtubules turn towards the front of the cell due to centrosomes and Golgi Complexes facing towards the cell’s front
These are more stable
Rac-GTPase is able to inhibit stathmin (a MT depolymerizing and sequestering protein) tp further stabilize the tublin
ALSO inhibits actin polymerization
What kind of movement do stable microtubules prefer?
Movement towards the front of the cell’s leading edge due to specific amino acid post-translational modifications.
these mods localize their stability by delivering motility factors
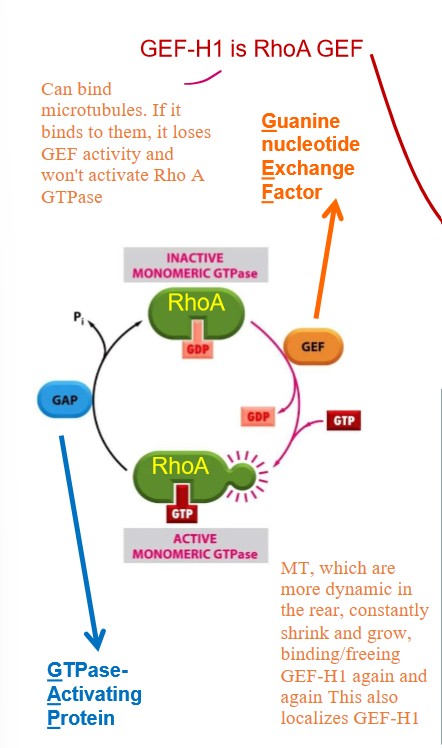
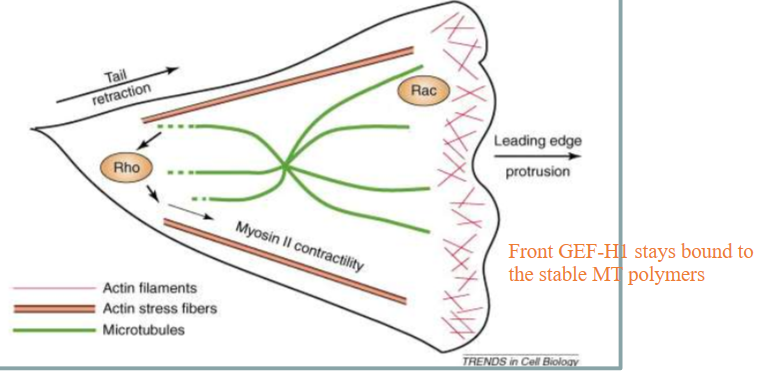
How SPECIFICALLY do microtubules regulate Rho GTPase?
Microtubules bind to the GEF of Rho-GTPase: GEF-H1. This prevents Rho-ATPase from being able to exchange GDP and bind to GTO
GEF-H1 also prevents MT depolymerization/catastrophe when bound to MT and inhibited
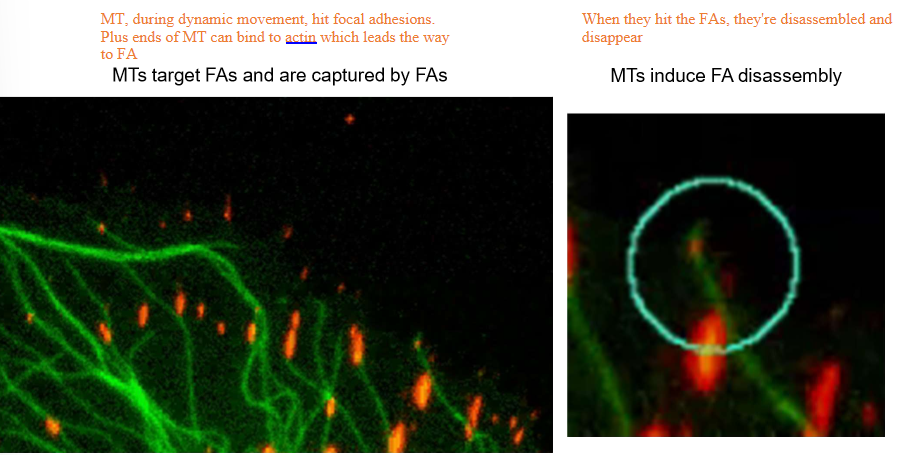
How do microtubules control focal adhesions?
By literally knocking into them
When MTs hit focal adhesions with their plus ends, they can bind to actin and dissociate the focal adhesions, making them disassemble and disappear
The depolymerizing microtubules will hit focal adhesions in the back of the cell, causing them to retract. The front, bound by GEF-H1, will not accidentally hit the focal adhesions.
Succinct: How do microtubules control directional migration?
Motility factors are moved towards the front leading edge of the cell
Focal adhesion dynamics are controlled
Rho-GTPase are regulated
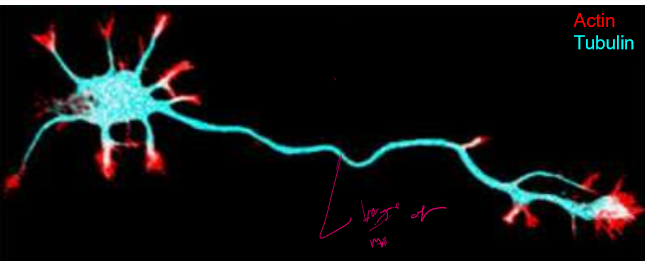
How do microtubules help determine the chape of neurons and protect them?
Axons are long, skinny, and stabilized by microtubules that end at their plus ends. These along with actin concentrated tips allow growth
Who are MAP2/ tau Family proteins?
They’re microtubule stabilizers that work analogous to tropomyosin.
MAP2 features small binding and a long extension domain. It’s used to stabilize dendrite microtubules. It’s extension is 25 nm
tau, with a much shorter extension region, is used to stabilize axons
Their extensions are flexible and allow for crosslinking of microtubules.
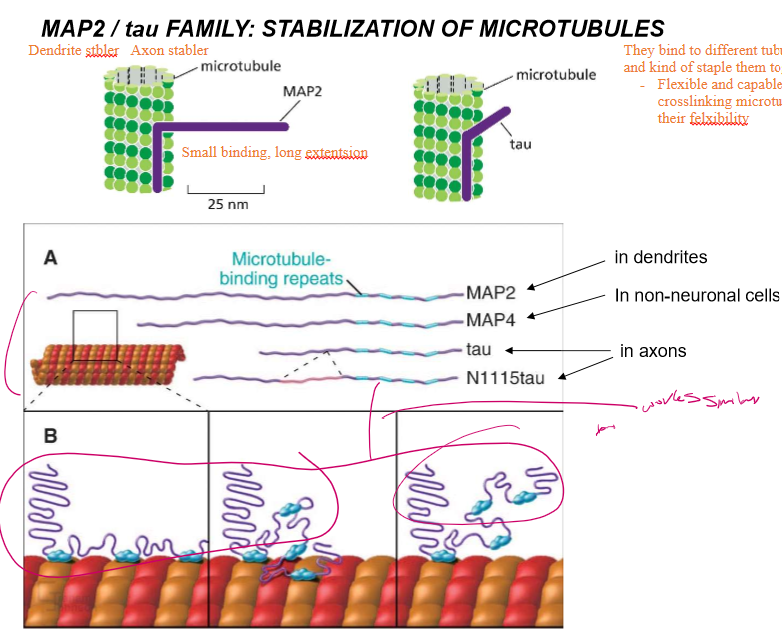
What do the extensions of MAP2 and tau allow the proteins to do?
Crosslink microtubules
What happens when MAP2 and tau are overexpressed?
The microtubules get EXCESSIVELY organized
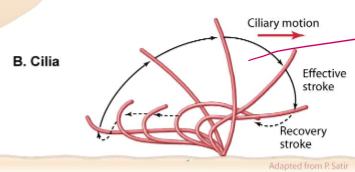
How do microtubules dictate the motility of cilia?
They regulate ciliary motion with power stroke motions akin to an Olympic swimmer. They be stroking.
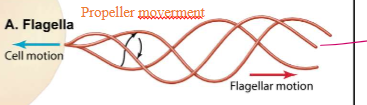
How do microtubules dictate the moevement of flagella?
They follow propeller-type movements that push the cells in space
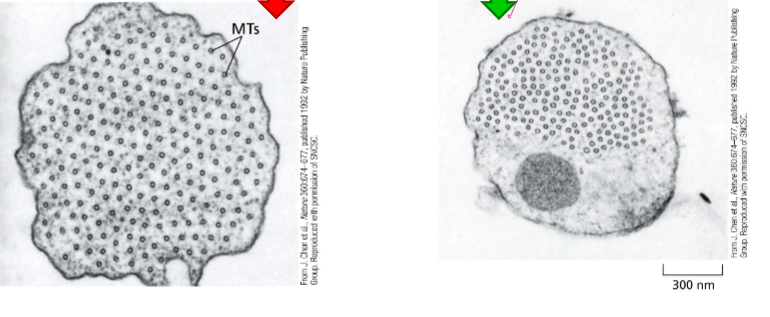
Describe the centrosome
Centrioles
Mature centriole and daughter centriole are made up of 8 triplets of MTs
Pericentriolar material (PCM)
gamma tubulin nucleates MTs here
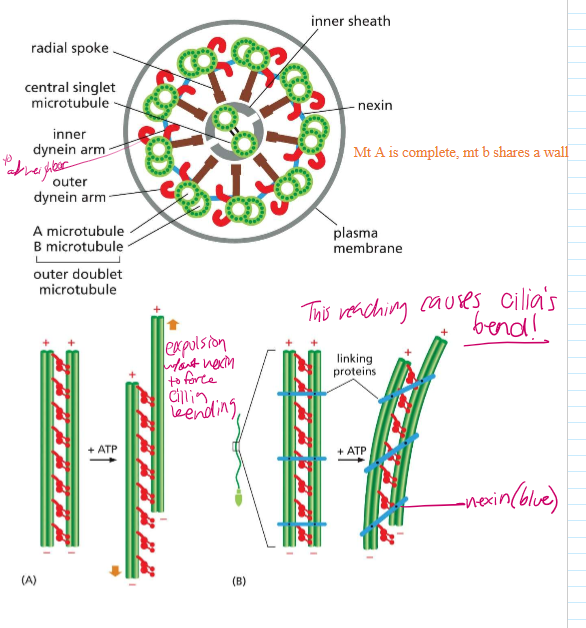
Describe the structure of Cilia and Flagella
axoneme are at the base structure of cilia, which are then extended to form the cilia. They and flagella are organized similarly
Flagella and cilia are organized into doublets with 2 singlet MTs in the middle. On their outsides sit dynein.
Mother centrioles act as the basal bodies of cilia and flagella.
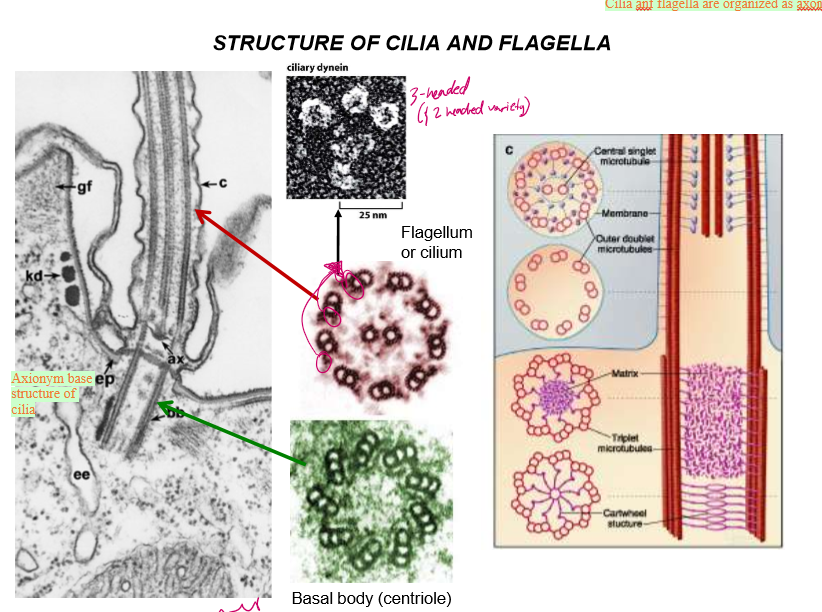
Take a closer look: how are cilia and flagella structured, and how does that affect their movement
Microtubule doublets share one wall
Nexin links neighboring microtubules together, forcing them to bend when dynein move along the MT.
Without nexin, the MT would expel each other without creating a bending force in the cilia.