Lab K: Introduction to Polymers
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
16 Terms
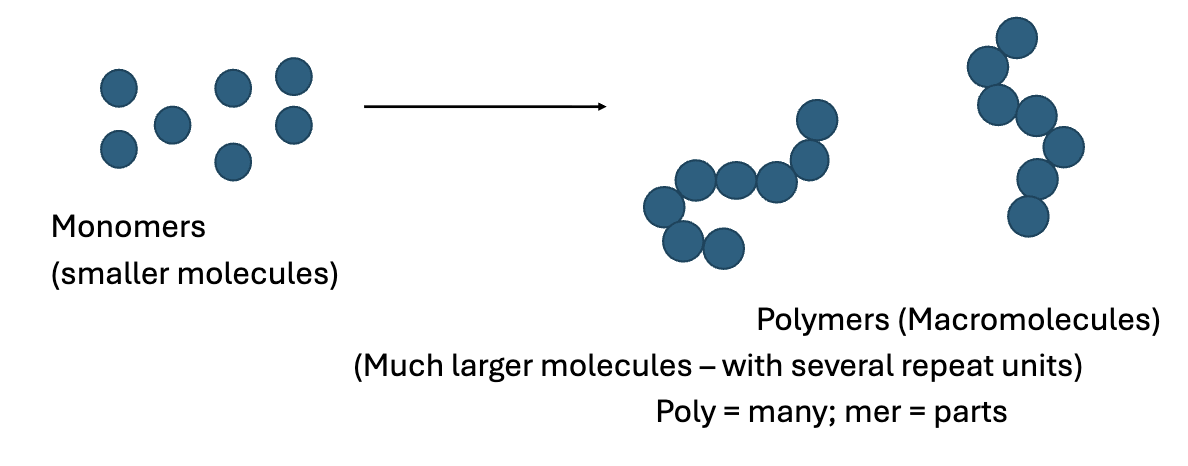
What are polymers?
Very large molecules that form when several monomer units join together to form long chains.
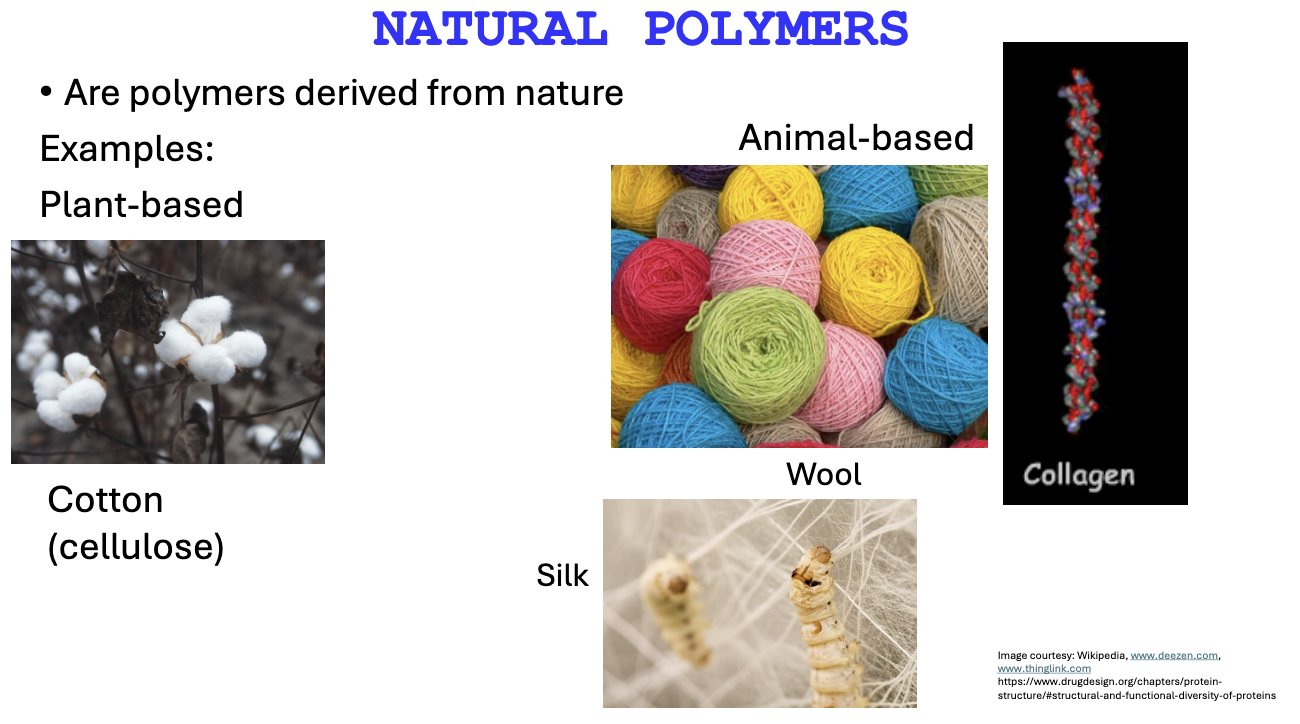
What are some examples of natural polymers?
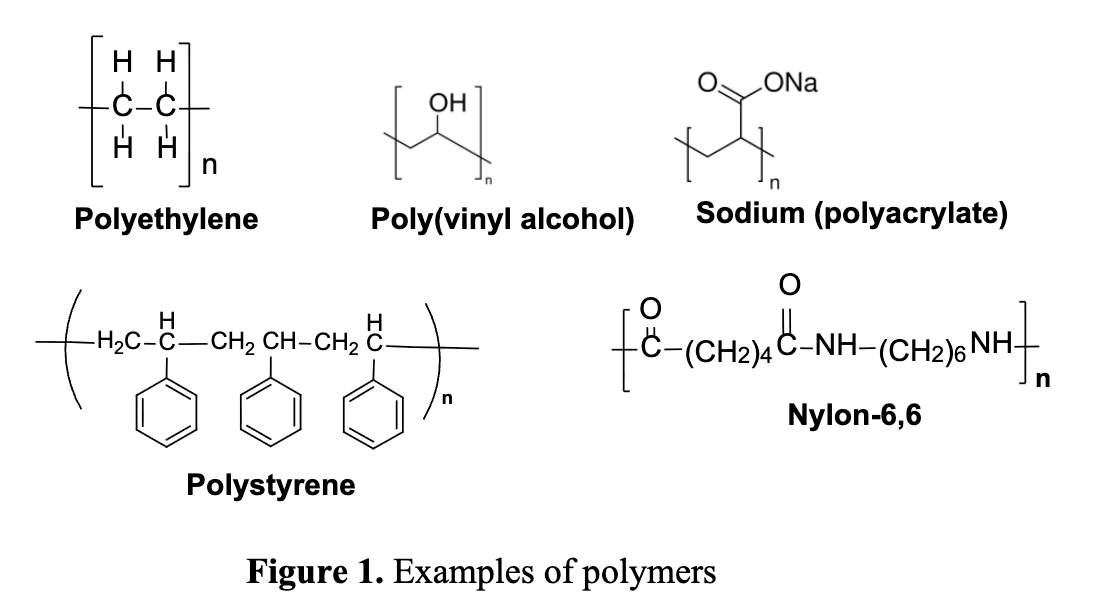
What are some artificial polymers?
Polyethylene: most common plastic used for packaging.
Nylon: man-made synthetic fiber - first produced in 1935 by Walllace Carother’s.
Polystyrene: synthetic aromatic polymer used in food service packaging industry.
Polyvinyl chloride (PVC)
Draw the structures of examples of polymers
What are 2 methods of polymerization?
ADDITION (chain growth) polymerization: ex. polystyrene, PVC
Involves addition of monomer units w/o loss of smaller molecules.
Monomers must contain double or triple bonds.
CONDENSATION (step growth) polymerization: ex. Nylon-6,6, Nylon-6,10
Involves reaction b/w 2 monomer units w/ reactive end functional groups w/ loss of small molecules e.g. water, methanol, HCl
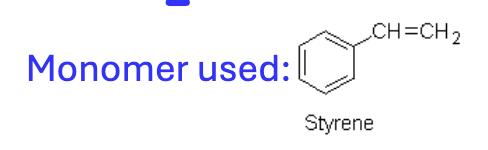
What is the monomer used in synthesis of polystyrene?
Styrene
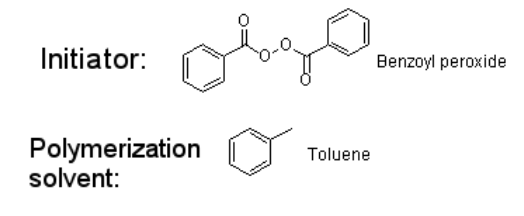
What is the initiator and polymerization solvent?
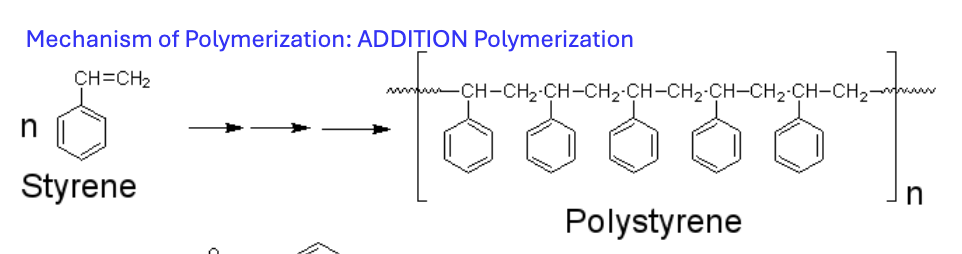
What is the mechanism of polymerization (ADDITION polymerization)?
What are the properties and applications of polystyrene?
Properties:
Non-polar
Naturally transparent
Lightweight
Great electrical insulator
Applications:
Disposable cups
Food packaging
Optical lenses
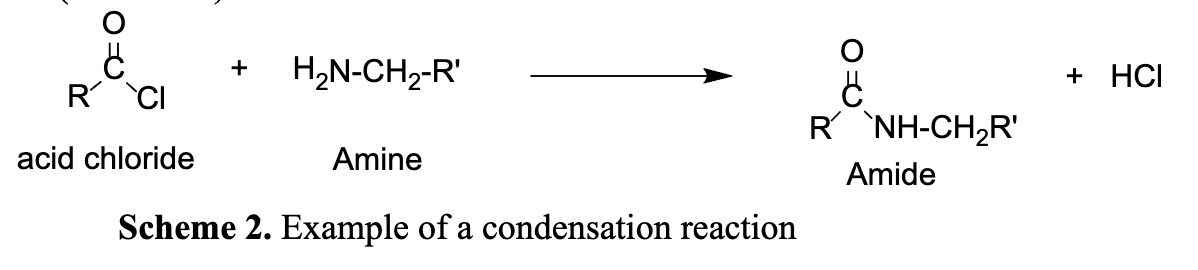
Draw the reaction scheme of a condensation reaction
Draw the reaction scheme for synthesis of Nylon-6,6
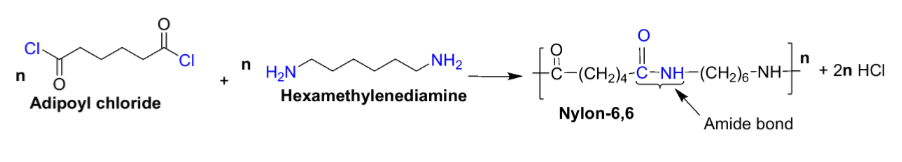
What are the monomers used for synthesis of Nylon-6,6?
Adipoyl chloride
Hexamethylenediamine
What are the physical properties and applications of Nylon-6,6?
Physical properties:
Strength, durability, high thermal stability
Applications:
Sports equipment
Industrial fabrics: tire cords, conveyor belts, seat belts
Apparel: swimsuits, jackets
Carpets, rugs
Automotive industry
Draw the reaction scheme for synthesis of Nylon-6,10
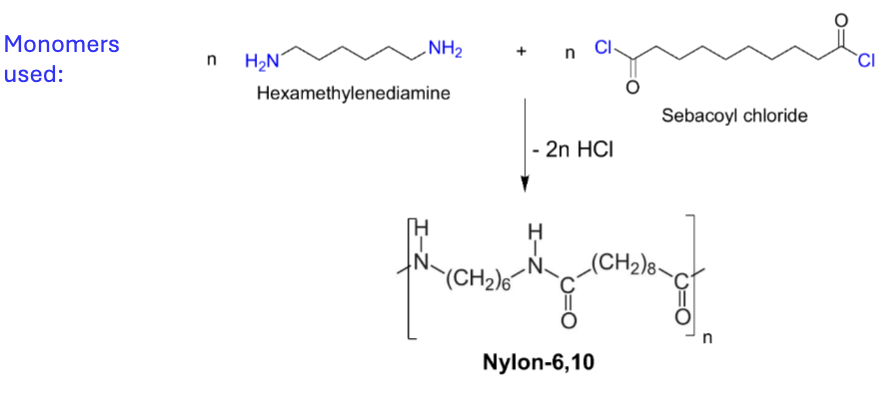
What are the physical properties and applications of Nylon-6,10?
Physical properties:
Good chemical resistance, low
Moisture absorption
Applications:
Electrical insulation
Medical tubing
Recreational sports equipment
Safety hazards?
Adiopyl chloride/hexane is a flammable liquid.
Hexamethylenediamine/sodium hydroxide is corrosive.