SN1 vs SN2 vs E1 vs E2
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
25 Terms
Sn1 Reaction stereochemistry
substitution with a mix of retention and inversion @ stereocenter

Sn1 Rate Law
sensitive to the concentration of the substrate ONLY
nucleophile ID and concentration does not matter
FIRST Order
Rate = k [R-X]
Sn1 Substrate preference
fastest for tertiary, slowest for primary
Sn1 Mechanism
STEPWISE
leaving group leaves (slow step), forming a carbocation intermediate
carbocation intermediate is attacked by nucleophile (fast step)
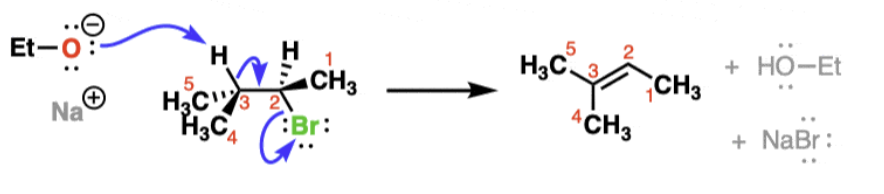
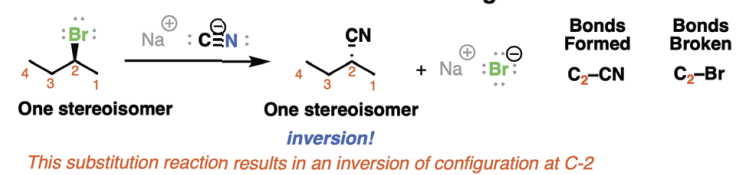
Sn2 Stereochemistry
substitution occurs with inversion of configuration at chiral centers
Sn2 Rate Law
rate is sensitive to concentration of substrate AND nucleophile
Rate = k [R-X][Nu-]
second order
Sn2 Substrate
slowest with tertiary, fastest for primary/methyl (steric hindrance repels nu-)
bulky groups slow down backside attack
Sn2 Mechanism
1-step backside attack
results in inversion of stereochemistry
substitution, nucleophilic, bimolecular
what solvent works best for Sn1?
polar protic (have H+ on electroneg. atom, lets them bond to cations and anions)
ex:
alcohols (R-OH)
methanol (CH3OH)
ethanol (CH3CH2OH)
H2O
What solvent favors Sn2?
polar aprotic — Nu- needs to be readily available to push leaving group
ex:
DMSO (dimethyl sulfoxide)
acetyl nitrile (CH3CN)
acetone (C3H6O or CH3COCH3)
dimethylformamide (HCON(CH₃)₂)
What kind of nucleophile do Sn1 prefer?
weak - generally neutral
What kind of nucleophile does Sn2 prefer?
strong (generally bearing a negative charge)
Comparing Sn1 vs Sn2: step 1
Identify leaving group
halogens (Cl, Br, I) or tosylates/mesylates (OTs, OMs)
if acid is present, look for alcohols (OH)
Comparing Sn1 vs Sn2: step 2
inspect the carbon that the leaving group is attached to
if the C is tertiary —> Sn1 (not Sn2 b/c steric hindrance)
if the C is primary —> Sn2 (not Sn1 b/c carbocation would be unstable, except for in the case of resonance stabilization)
Comparing Sn1 vs Sn2: step 3
examine nucleophile
negative nucleophile —> generally Sn2
neutral nucleophile (H2O or R-OH) —> generally Sn1
Comparing Sn1 vs Sn2: step 4
check solvent
polar aprotic (DMSO, acetone, acetonitrile, DMF) —> generally Sn2
polar protic (H2O, ROH) —> generally Sn1
E1 rate law
unimolecular
depends on concentration of SUBSTRATE
rate = k [substrate]
What is the “big barrier” for E1?
forming a carbocation (stability)
tertiary > secondary > primary
E1 vs E2: is there a strong base?
no strong base = E1
strong base present/req. = E2
Stereochemistry requirements for E1
none
E2 reaction rate law
bimolecular
rate = [base][substrate]
depends on BOTH substrate and base!
“big barrier” for E2
no big barrier
stereochemistry of E2 reaction
leaving group must be anti to hydrogen removed
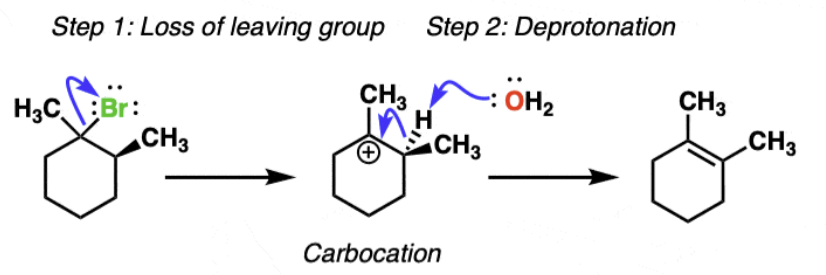
E1 mechanism rate-determining step =?
unimolecular rate determining step = loss of leaving group to form a carbocation
proceeds faster when a more stable carbocation can be formed
Steps:
loss of leaving group
deprotonation
E2 mechanism
1-step, concerted reaction
strong base simultaneously removes a beta-hydrogen, a pi bond forms between the two carbons
leaving group departs, leading to an alkene