Chem flashcards (exam 2)
1/40
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
41 Terms
What is the charge of the magnesium Ion?
+2
Natural gas consists primarily of methane, CH4. How many hydrogen atoms are in one methane molecule?
4
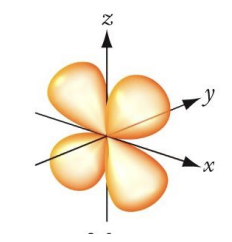
This image represents?
d orbital
Which element would you expect to have chemical properties most similar to Br?
I
NO2 makes smog brown, what is the correct name of NO2
Nitrogen dioxide
Compared to a single 3p orbital, a single 4p orbital has ________ energy, _________ size, and ________ shape.
Higher, Larger, the same
Select the element with electron configuration [Kr]5s² 4d³
Nb
Sodium sulfide is used in paper making, what is the formula for sodium sulfide?
Na2S
Octane, C8H18, is a component of gasoline. What is the molar mass of octane?
114.22g/mol
When the principle energy level (shell) is n = 2, what are/is the possible subshell(s)
2s and 2p subshells
In the quantum mechanical model, what do atomic orbitals represent?
the probability that an electron is found at a particular location
Elements with the highest first ionization energies are found in the ________ region of the periodic table.
upper right
large Electron affinity
an element that accepts an electron most readily
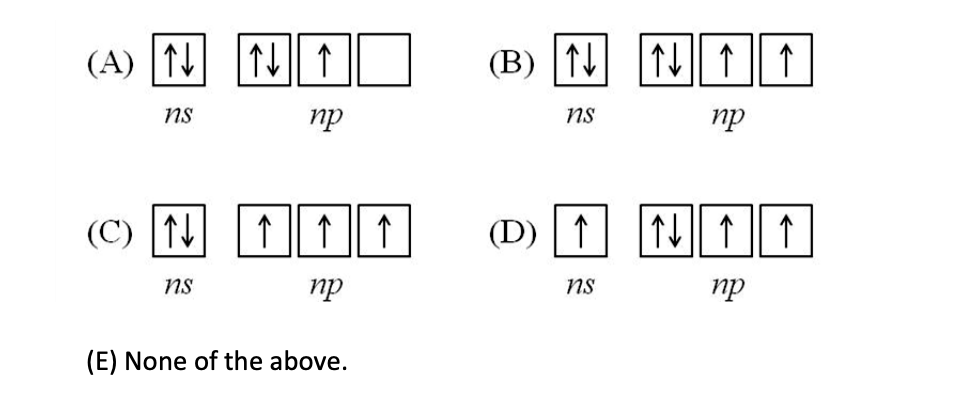
. Which orbital diagram presents the ground state configuration of the valence electrons of a Group 5A element such as nitrogen?
C
Which transition in a hydrogen atom will result in the emission of the highest energy photon?
n=3 to n=1
6. How many valence electrons are in an atom with the electron configuration [Ar]4s²3d^104p³?
5
the effective nuclear charge of an atom is less than the actual nuclear charge due to?
shielding
how does atomic radius change as you move across the periodic table?
decreases moving left to right, increases top to bottom.
which factor contributes to the tendency of main group atoms to form monatomic ions with predictable charges?
Nonmetals can gain enough electrons to fill their outermost s- and p-subshells
NASA’s Europa Clipper probe contains a canister of perfluorotributylamine, Cv12Fv27N. How many moles of fluorine are in 0.25 moles of perfluorotributylamine?
6.8mol
What is the electron configuration for the bromide ion Br –?
1s²2s²2p^63s²3p^64s²3d^104p^6
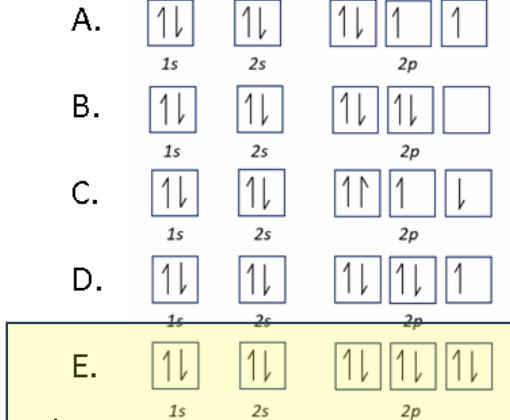
Which orbital diagram accurately represents an oxide ion O^2-
E
Which of the following is a molecular compound? A. Li2S B. C2H6 C. H2 D. Ca(OH)2 E. (NH4)2SO4
B. C2H6
. Nitrite ions are found in lunchmeats. What is the formula for the nitrite ion?
NO2 –