Rates of Reactions
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
18 Terms
Equation for Rate
Change in conc/ time
When is rate the fastest
conc of reactants is highest
When does rate start slowing down?
When the concentration of reactants decreases
When is Rate 0
once reactants are used up
How are rates calculated?
by measuring amount of reactants used up or amount of product formed over period of time
What does the gradient represent
the initial rate of reaction
What do we do to find reaction at a given time
draw a tangent at that given time
Factors affecting rates of reactions
Concentration of solution
Surface Area
Temperature
Catalyst
Pressure
Light in some
What is the collision theory
For a chemical reaction to take place, reacting molecules must collide effectively.
What is the reaction a measure of?
how frequently successful collisions occur
For collisions to be effective, molecules must collide in the
correct orientation
sufficient energy
Define activation energy
minimum energy needed for a reaction to occur
Any __________ that increases chance of ___________ collisions, also increases ___________________.
factor
effective
rate of reaction
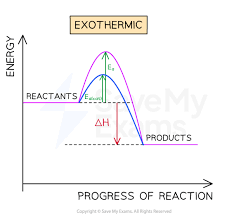
Exothermic
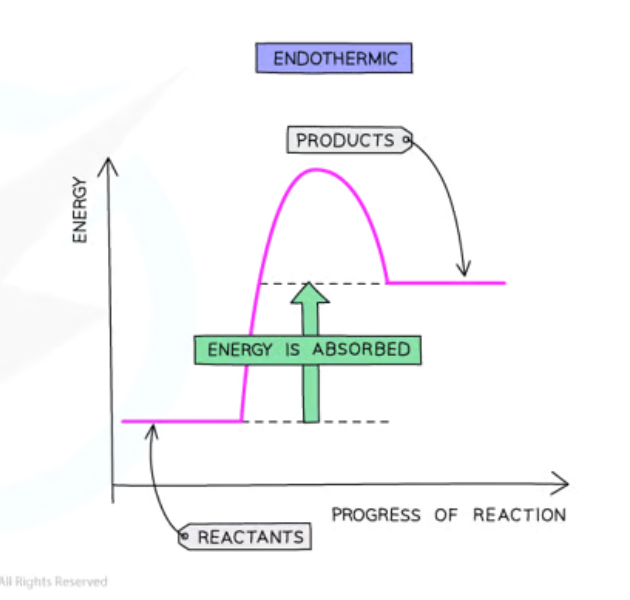
Endothermic
Exothermic Reactions
Products have less energy than reactants
Excess energy is lost from reaction as heat
∆H is negatibe
Endothermic Reactions
Energy of products is more than reactants
Heat is taken in from surroundings
∆H is positive