Introduction to Organic
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
25 Terms
Organic chemistry summary
The chemistry of carbon containing compounds except diamond, graphite, carbonates CO and CO2
Each carbon forms 4 covalent bonds
Molecular formula definition (must know Mr)
The actual number of atoms of each element present in a molecule e.g. C4H8
Empirical formula definition
The simplest whole number ratio of atoms of each element present in a molecule e.g. CH2
What is a structural formula?
Shows how the atoms in a molecule are arranged e.g. pentane is CH3CH2CH2CH2CH3
What is a displayed formula?
Shows all the atoms and bonds in a molecule
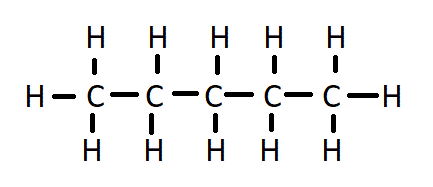
What is skeletal formula?
Shows the shape of the carbon skeleton
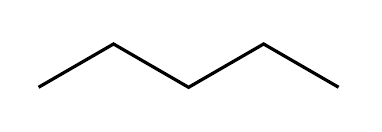
Homologous series definition
A family of compounds containing the same functional group and having the same general formula but having a different carbon chain length- each successive member has an extra CH2
e.g. alcohols all have -OH (functional group)
Functional group definition
An atom or group of atoms which gives an organic compound its particular chemical properties
How to find the general formula of a compound?
Count up the number of carbon and hydrogen atoms
CnH2n- either subtract or add numbers to fit this formula
Suffixes of functional groups in the homologous series
Alkene -ene
Alkane -ane
Amine -amine
Alcohol -ol
Ketone -one
Aldehyde -al
Nitrile -nitrile
Acyl chloride -oyl chloride
Ester -oate
Carboxylic acid -oic acid
Hydrocarbons definition
Compounds containing carbon and hydrogen atoms only
Saturated (hydrocarbons) definition
Contain single carbon-carbon bonds only e.g. alkanes
Unsaturated (hydrocarbons) definition
Contain one or more carbon-carbon double bonds e.g. alkenes
What are different types of hydrocarbons?
Aliphatic: contain carbons in chains
Alicyclic: contain carbons in rings
Aromatic: contain a benzene ring
How to name branched alkanes?
First find the longest unbranched (continuous) chain and name the stem
Identify the alkyl groups as side chains- these are added to the stem as a prefix
Give the position of the side chain by numbering the main chain starting from the end which gives the lowest number
How to name branched alkanes if there are more than one side chains?
Name in alphabetical order
Number the main chain from the end which gives the lowest combination of numbers
If alkyl groups are the same- prefix with di- tri- tetra-
Each alkyl group is given its own number
Numbers are separated from names by hyphens, and numbers
How to name compounds?
Stem- how many carbons there are in the longest chain
Prefix- before the stem- position of side chains and/or functional groups
Suffix- after the stem- tells us functional group present
3-methylpentane
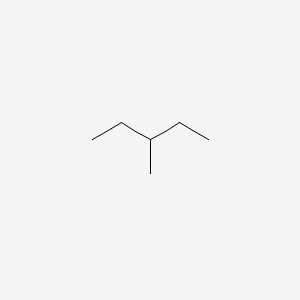
How to find the stem and alkyl group from the number of carbons in longest chain?
Stem: meth, eth, prop: increases as a carbon atom is added
Alkyl group (prefix) if the length of the side chain is 1, it would be methyl, if it is 2 it would be ethyl, propyl, butyl
How to name cyclic alkanes?
General formula CnH2 (functional group isomers of alkenes)
The stem is cyclo- followed by the name of the longest continuous carbon ring
The positions of alkyl groups are shown by numbers (if there is just one side chain, a number is not needed)
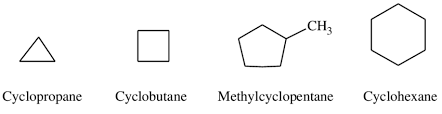
Structural isomers definition
Compounds with the same molecular formula but a different structural formula
What are the 3 types of structural isomerism?
Chain, position and functional group
What are chain isomers?
They have the same molecular formula but their structures have the hydrocarbon chain arranged differently e.g. C4H10 can be butane or methylpropane
What are position isomers?
They have the functional group in a different position (the numbers in the name will be different) e.g. C3H8O can be propan-1-ol or propan-2-ol
What are functional group isomers?
They have the same molecular formula but a different functional group (the suffix in the name will be different) e.g. pent-1-ene and cyclopentane
Stereoisomerism definition
Compounds with the same structural formula but a different arrangement of atoms in space