Group 7, Halogens
1/80
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
81 Terms
fluorine colour and state at room temperature
pale yellow gas
chlorine colour and state at room temperature
pale green gas
bromine colour and state at room temperature
brown-orange liquid
iodine colour and state at room temperature
grey solid
trend in boiling point down group 7
increases down the group
why does boiling point increase down group 7
van der waals forces increase due to increasing size and relative mass of atoms,
physical state goes from gas at the top of group 7 to solid at the bottom
trend in electronegativity down group 7
decreases down the group
define electronegativity
the power an atom has to attract a pair of electrons to itself from a covalent bond
why does electronegativity decrease down group 7
atomic radius increases, distance between positive nucleus and bonding electrons increases = less attraction
more shielding down group 7
what do more reactive halogens displace in displacement reactions
more reactive halogens will displace less reactive halide ions
trend in reactivity down group 7
decreases down group 7
why does reactivity decrease down group 7
atomic radius increases, less ability to attract electrons compared to atoms with a smaller radius
trend in oxidising power down group 7
less oxidising as we go down,
shown by reacting halogens with halide ions
why does oxidising power decrease down group 7
atoms with a smaller radius attract electrons easier,
for a reaction to occur, an electron must be gained,
atomic radius increases down group 7, so harder to attract an electron
when will a halogen displace a halide from solution
if the halide is LOWER in the periodic table,
more reactive halogens displace less reactive halides
in terms of electrons, what must occur for a displacement reaction to occur
an electron must be gained
does displacement occur when chlorine water is added to potassium chloride solution
no reaction,
both have Cl which is the same reactivity
is there a displacement reaction when chlorine water is added to potassium bromide solution
yes,
Cl2 + 2Br → 2Cl- + Br2
what can be observed from the displacement reaction between addition of chlorine water (almost colourless) to potassium bromide solution (colourless)
orange solution, as Br2 is made
is there a displacement reaction between potassium iodide solution (KI, colourless) and chlorine water
yes
Cl2 + 2I- → 2Cl- + I2
what is observed during displacement reaction between potassium iodide solution and chlorine water
brown solution, as iodine (I2) is made
is there a displacement reaction between potassium chloride solicitor and addition of bromine water (orange)
no reaction, bromine is less reactive than chlorine
is there a displacement reaction between potassium bromide solution and bromine water
no reaction, both contain bromine which are the same reactivity
is there a displacement reaction between potassium iodide solution and bromine water? what is observed?
yes,
brown solution is formed as I2 is made
Br2 + 2I- → 2Br- + I2
bromine is more reactive than iodide
is there a displacement reaction between potassium chloride solution and addition of iodine solution (brown)
no reaction, iodine is less reactive/oxidising than chloride ion
is there a displacement reaction between potassium bromide solution and iodine solution
no reaction, iodine is less reactive/oxidising than bromide
is there a displacement reaction between potassium iodide solution and iodine solution
no reaction, both contain iodine which have the same reactivity
which reaction is bleach made from
disproportionation reaction
mixing chlorine and sodium hydroxide will form sodium chlorate (I) solution = bleach
what is a disproportionation reaction
a redox reaction where an element is both oxidised and reduced
reaction to make bleach?
chlorine + sodium hydroxide → sodium chlorate (I)
2NaOH (aq) +Cl2 (g) → NaClO (aq) + NaCl (aq) + H2O (l)
which element has been reduced and oxidised in the reaction to make bleach and how
chlorine
reactant: Cl2, oxidation state of 0
product: NaClO, chlorine has an oxidation state of +1
product: NaCl, chlorine has an oxidation state of -1
uses of sodium chlorate (I), bleach
treating water
bleach paper and fabrics
cleaning reagent (bleach)
importance of water sterilisation
stop outbreaks of water borne diseases
what do we add to water to sterilise it
chlorine
how does adding chlorine to water sterilise it
chlorate (I) ions (ClO-) kill bacteria,
useful in drinking water and pools
reaction between water and chlorine
H2O (l) + Cl2 (g) → 2H+ (aq) + Cl- (aq) + ClO- (aq)
how is the reaction between chlorine and water a disproportionation reaction
chlorine is oxidised and reduced
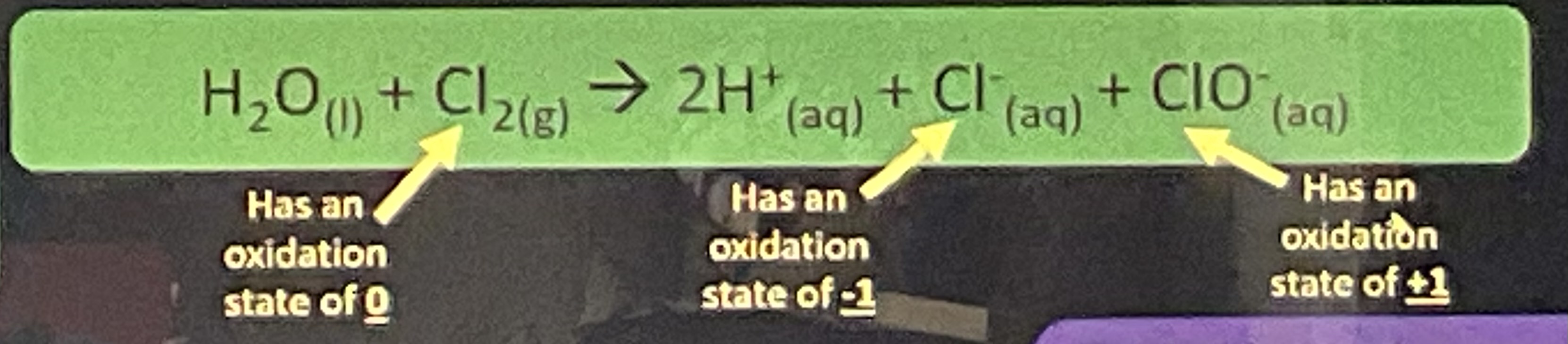
what can decompose chlorinated water
sunlight
reaction between water and chlorine in strong sunlight
2H2O (l) + Cl2 (g) → 4H+ (aq) + 2Cl- (aq) + O2 (g)
issue with sunlight decomposing water and chlorine? how is this solved?
no ClO- is made, so bacteria is not killed (no active ingredient)
in swimming pools, disease can spread as sunlight is concentrated
so we constantly top up the chlorine in swimming pools
advantages of chlorinating drinking water
destroys microorganisms which cause disease
long lasting so reduce bacteria build up further down the supply
reduces growth of algae that discolours water and can give it a bad smell and taste
small amounts of chlorine is used, so risk of cancer is very LOW
disadvantages of chlorinating drinking water
chlorine gas is toxic and irritates the respiratory system, in high concentrations
liquid chlorine causes severe chemical burns to the skin, in high concentrations
chlorine can react with organic compounds present in water to make chloroalkanes - have links to causing cancer, but risk of not chlorinating water could lead to a cholera epidemic
how are halide ions reducing agents
they lose an electron in reactions
define reducing agent
oxidised themselves, lose electrons
trend in reducing power of halide ions down the group
reducing power increases down the group
F- , weakest
Cl-
Br-
I-, strongest
why does reducing power of halides increase down group 7
ionic radius increases
distance between nucleus and outer electrons become larger and there’s more shielding, weakening the attractive force
outer electron is lost more readily (reducing agents lose an electron during reactions)
2 tests to prove reducing power of halide ions
1 reaction with sulfuric acid
2 reaction with silver nitrate
reaction between sodium chloride and sulfuric acid
H2SO4 + NaCl → NaHSO4 + HCl
what is observed from the reaction between sulfuric acid and sodium chloride
white, steamy fumes, from HCl gas produced
why isn’t the reaction between sodium chloride and sulfuric acid a redox reaction
oxidation state of sulfur remains the same, +6
so, NaHSO4 is not a reduction product
why is there no further reaction between chloride ions and sulfuric acid after production of NaHSO4
Cl- is not a strong enough reducing agent to reduce sulfur
reduction products of sulfur
SO2 (+4)
S (0)
H2S (-2)
first reaction of sodium bromide with sulfuric acid
HS2SO4 + NaBr → NaHSO4 + HBr (displacement, not redox)
second/redox reaction between bromide ions and sulfuric acid, using products from the first x
2HBr + H2SO4 → Br2 + SO2 + 2H2O
what can be observed during the reduction of sulfur by bromide ions
Br2 gas produced = orange vapour
what is oxidised and reduced in the reaction between HBr and H2SO4, state oxidation state
Br- ions are oxidised
Sulfur in H2SO4 (+6) is reduced to SO2 (+4)

first reaction between sodium iodide with sulfuric acid
NaI + H2SO4 → NaHSO4 + HI (displacement)
second reaction of hydrogen iodide, HI, with sulfuric acid
2HI + H2SO4 → I2 + SO2 + 2H2O
what is oxidised and reduced between the reaction of hydrogen iodide and sulfuric acid
iodide ions are oxidised to I2
sulfur in H2SO4 (+6) is reduced to SO2 (+4)
further reduction equation between hydrogen iodide and sulfuric acid
6HI + H2SO4 → 3I2 + S + 4H2O
H2SO4 (+6) reduced to S (0)
what can be observed during this further reduction reaction of sulfuric acid
yellow solid, as sulfur (s) is produced
full reduction of sulfuric acid with hydrogen iodide/iodide ions
other equations here, then:
8HI + H2SO4 → 4HI2 + H2S + 4H2O
what is observed/smelt from the full reduction of sulfur using iodide ions
rotten egg smell, from H2S (g)
why does greater reducing power = longer reactions
halide is powerful enough to reduce more species
how to test for halides
1 add dilute nitric acid (HNO3)
2 add silver nitrate
3 confirm halide with ammonia solution
why do we add dilute nitric acid when testing for halides with silver nitrate
to remove any carbonite/sulfite impurities, so there’s no false result
simple ionic equation between Ag+ and halides
Ag+ (aq) + Cl- (aq) → AgCl (s)
Ag+ (aq) + Br- (aq) → AgBr (s)
Ag+ (aq) + I- (aq) → AgI (s)
what do these reactions produce, why is a precipitate formed
produce INSOLUBLE silver halides
halide test results after adding silver nitrate
AgF / F- = none
AgCl / Cl- = white
AgBr / Br- = cream
AgI / I- = yellow
why doesn’t AgF produce a precipitate
AgF is soluble
how do further tests confirm the halide present
(results when ammonia is added)
add ammonia,
AgCl dissolves in dilute ammonia
AgBr dissolves in concentrated ammonia
AgI is insoluble in any concentration of ammonia
test for group 2 cations
flame test used for a solid sample,
1 dip nichrome wire into HCl to clean
2 dip into sample
3 place loop into blue flame and observe colour
results for positive group 2 cation test, Ca2+, Sr2+, Ba2+
Ca2+ = dark red
Sr2+ = red
Ba2+ = green
can flame test be used for solutions? issue with this?
yes, it can be made into a solution and dipped into the loop, but will be difficult if the sample is insoluble
how to test for ammonium compounds with litmus paper + positive result
1 add sodium hydroxide and gently heat
2 if ammonium compound is present, ammonia gas is produced (alkaline)
3 damp red litmus paper turns blue is alkaline ammonia is produced
reaction between ammonium ions and hydroxide ions?

test for hydroxides with litmus paper, issues with test?
hydroxides are alkaline so will turn red litmus paper blue,
this does not fully confirm a hydroxide because red litmus turns blue for any alkaline
test for carbonates
HCl + carbonate → CO2 (g)
bubble through limewater = cloudy if CO2 gas present
test for sulfates
add HCl to remove any carbonates
add barium chloride, BaCl2
positive test = white precipitate
what forms the white precipitate in sulfate test
BaSO4 (s), barium sulfate - insoluble
Ba2+ (aq) + SO4 2- (Aq) → BaSO4 (S)
order of tests to prevent false positives
1 carbonates
2 sulfates
3 halides