7. transition metal titrations
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
10 Terms
titration is used to how out
how much oxidising agent is needed to exactly react w a quantity of reducing agent
if you know the concentration of either the oxidising agent or the reducing agent
you can use the titration results to work out the concentration of the other
transition metals have variable oxidation states
they are often rpesent in either the oxidising or reducing agent
transition metal colour change also makes them useful for titration
as it is easy to spot the end point
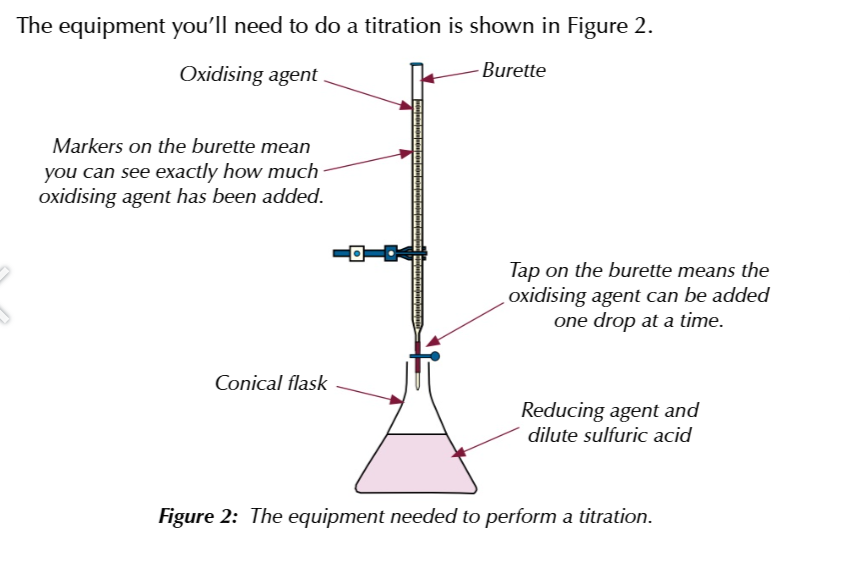
performing the titration:
first you measure out the quantity of the reducing agent (Fe2+/ C2O42-) using a pipette and put it in a conical flask
using a measuring cylinder, add 20cm3 od dilute sulfuric acids to the flask
this is in excess so you dont need to be exact
bthe acid is added to make sure there are plent of H+ ions to allow the oxidising agent to be reduced
now add the oxidising agent (potassium manganate 7) to the reducing agent using a burette, swirling the conical flask as you go
the pxidising agent you add reacts w the reducing agent, the reaction will continue untill all of the reducing agent is used up
the very next drop you add to the flask will give the mixture the colour of the oxidising agent
you could use a coloured reducing agent and a colourless oxidising agent then watch for the moment the colour dissapears in the flask
stop when the mixture in the flask just becomes tainted w the colour ox the oxidising agent (AKA end point) and record the volume of oxidising agent added
this is the rough titration
now you do some accurate titrations till you get results within 0.1 cm3 of eachother
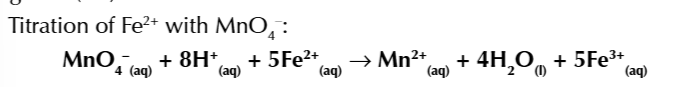
titration reaction of the reducing agent Fe2+ against the oxidising agent Manganate 7 (MnO4)
MnO4- + 8H+ + 5Fe2+ → Mn2+ +4H2O + 5Fe3+
titration of C2O42- with MnO4-
2MnO4- + 16H+ + 5C2O42- → 2Mn2+ + 8H2O + 10CO2
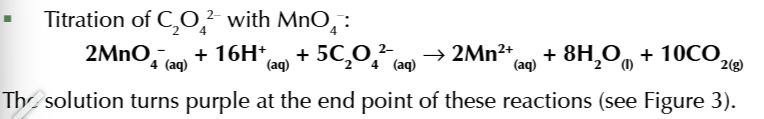
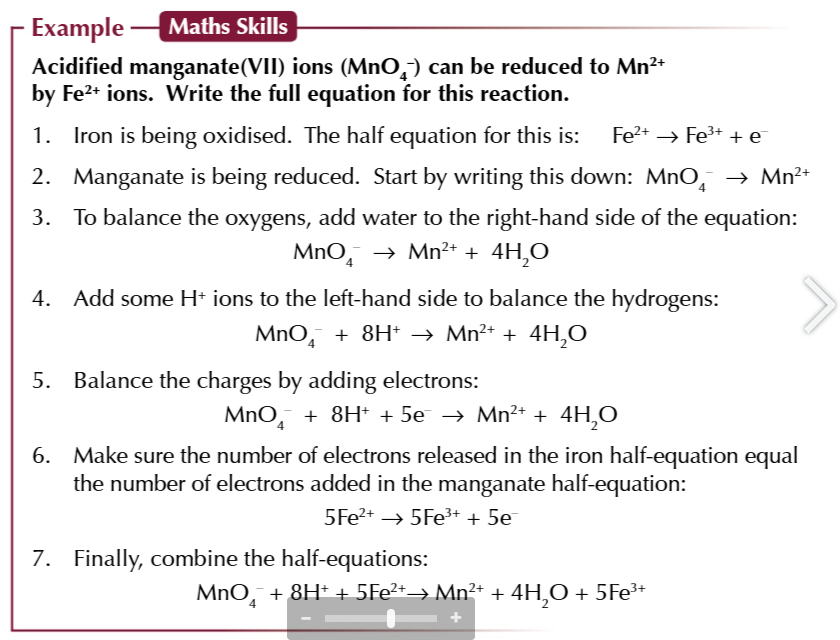
balanced under acidic conditions
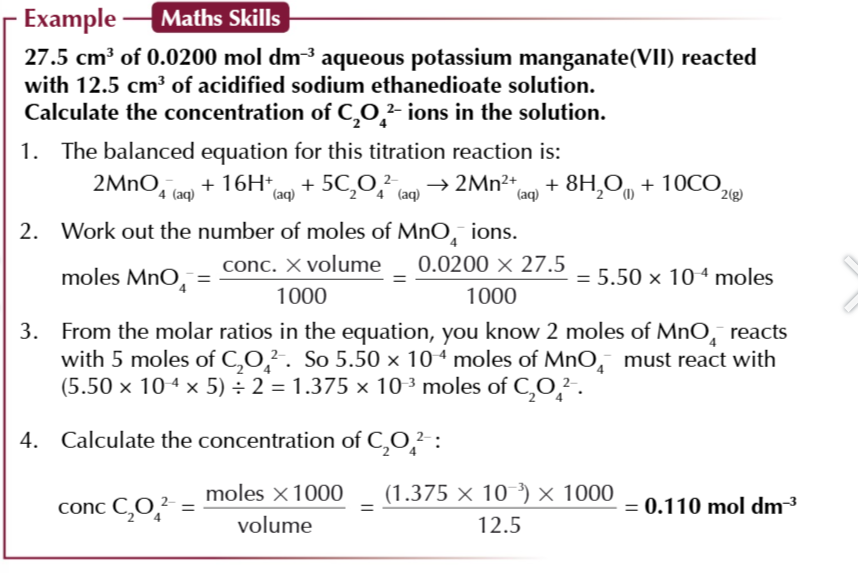
calculating the concentration of the reagent (oxidising/reducing):
write out a balanced equation for the redox reaction thats occured in the conical flask
decide what you already know and what you need to know
you’ll already know 2 volumes and the concentration of 1 reagent
you’ll need to find out the concentration for the other reagent
for the reagent you know both the concentration and volume for, calculate the number of moles
use the molar ratios in the balanced equation to find out how many moles of the other reagent were repsent in the solution
calculate the unknown concentration using conc = (mol × 1000)/vol (cm3)