periodic table
5.0(2)Studied by 30 people
Card Sorting
1/26
Last updated 4:39 PM on 1/3/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
27 Terms
1
New cards
the number of e- that can occupy the sublevels being filled in that period
What determines the length of each period?
2
New cards
2
1s
1s
What are the # of elements and sublevels for period 1?
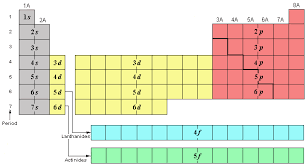
3
New cards
8
2s 2p
2s 2p
What are the # of elements and sublevels for period 2?
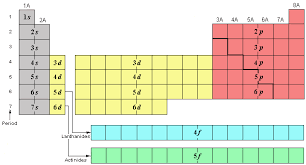
4
New cards
8
3s 3p
3s 3p
What are the # of elements and sublevels for period 3?
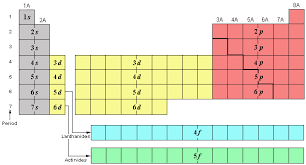
5
New cards
18
4s 3d 4p
4s 3d 4p
What are the # of elements and sublevels for period 4?
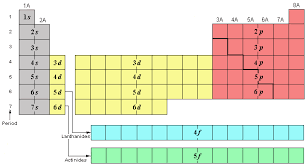
6
New cards
18
5s 4d 5p
5s 4d 5p
What are the # of elements and sublevels for period 5?
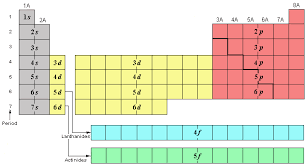
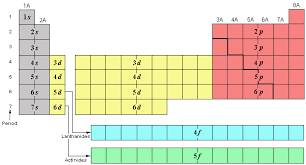
7
New cards
32
6s 4f 5d 6p
6s 4f 5d 6p
What are the # of elements and sublevels for period 6?
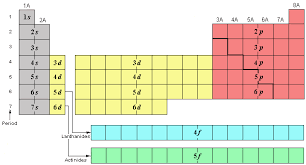
8
New cards
32
7s 5f 6d 7p
7s 5f 6d 7p
What are the # of elements and sublevels for period 7?
9
New cards
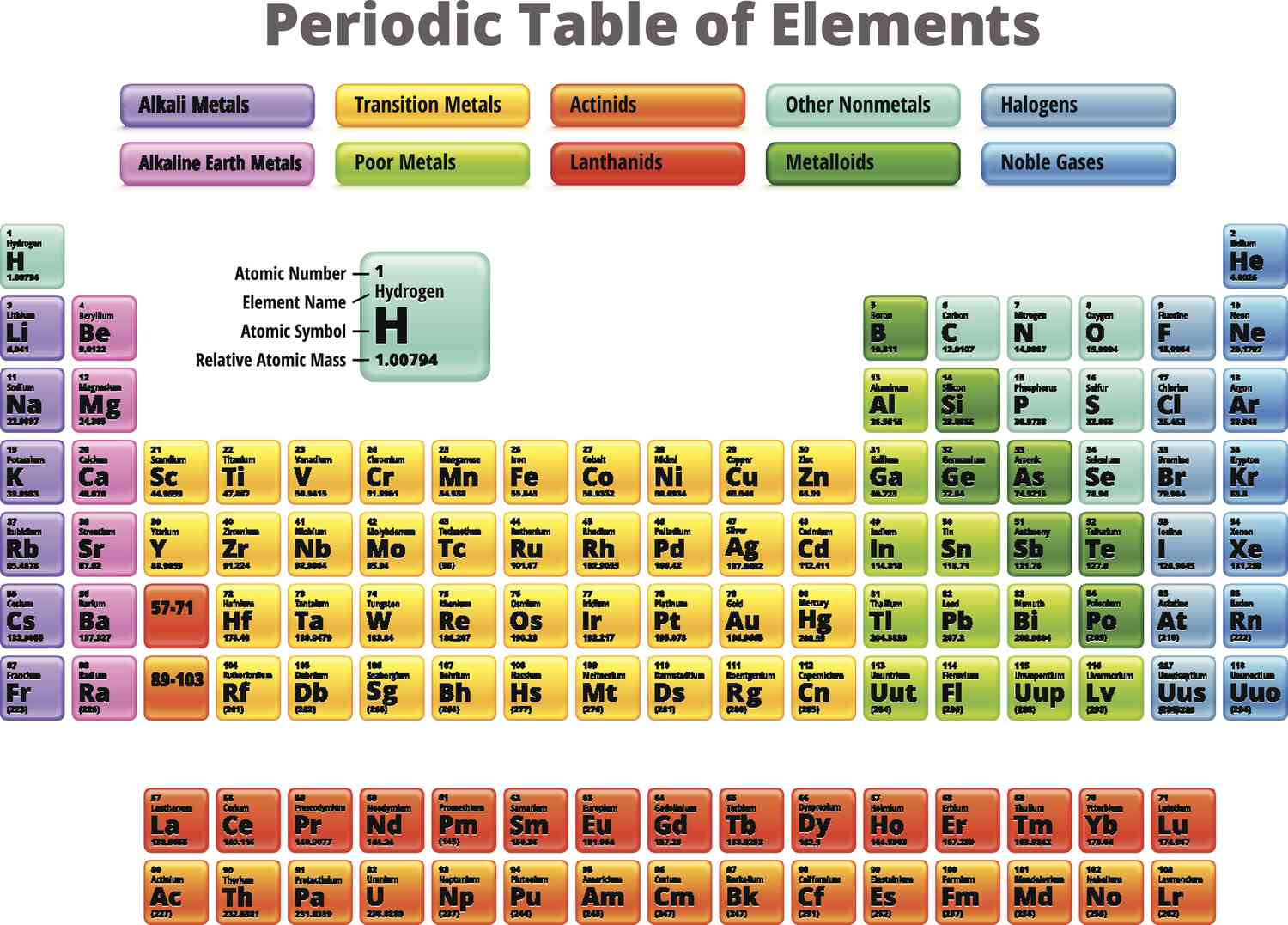
groups 1 & 2
what groups are the s-block elements?
10
New cards
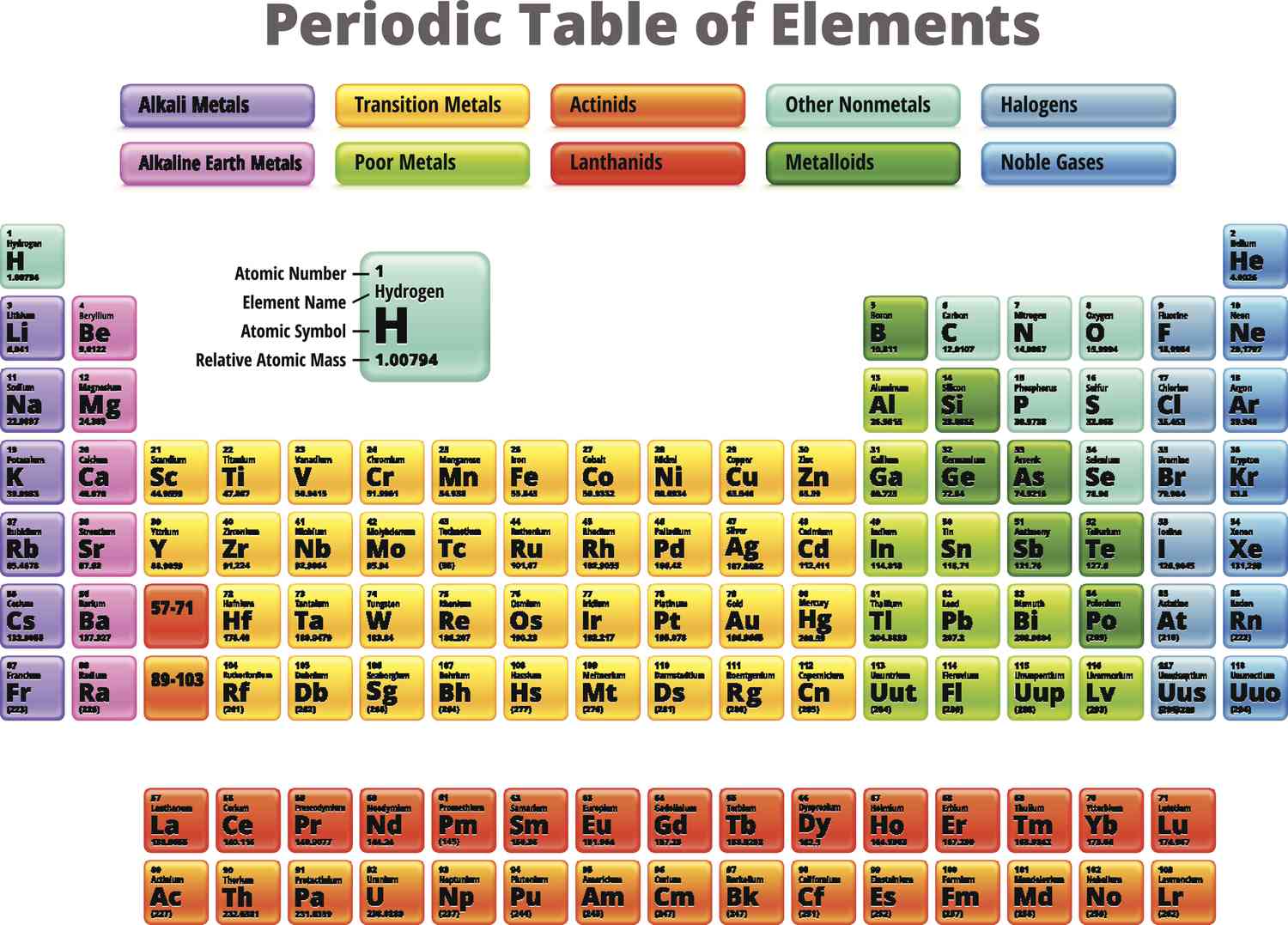
alkali metals
silvery appearance
soft enough to cut with knife
very reactive, must be stored in kerosene bc they will react with the water in the air
not found in nature as free elements, only in compounds
group 1
soft enough to cut with knife
very reactive, must be stored in kerosene bc they will react with the water in the air
not found in nature as free elements, only in compounds
group 1
11
New cards
alkaline earth-metals
harder, denser, & stronger than alkali metals
higher boiling point
less reactive but also not found in nature as a free element
group 2
higher boiling point
less reactive but also not found in nature as a free element
group 2
12
New cards
H & He
unique, does not share the same properties of group 1
13
New cards
groups 3-12
what groups are the d-block elements?
14
New cards
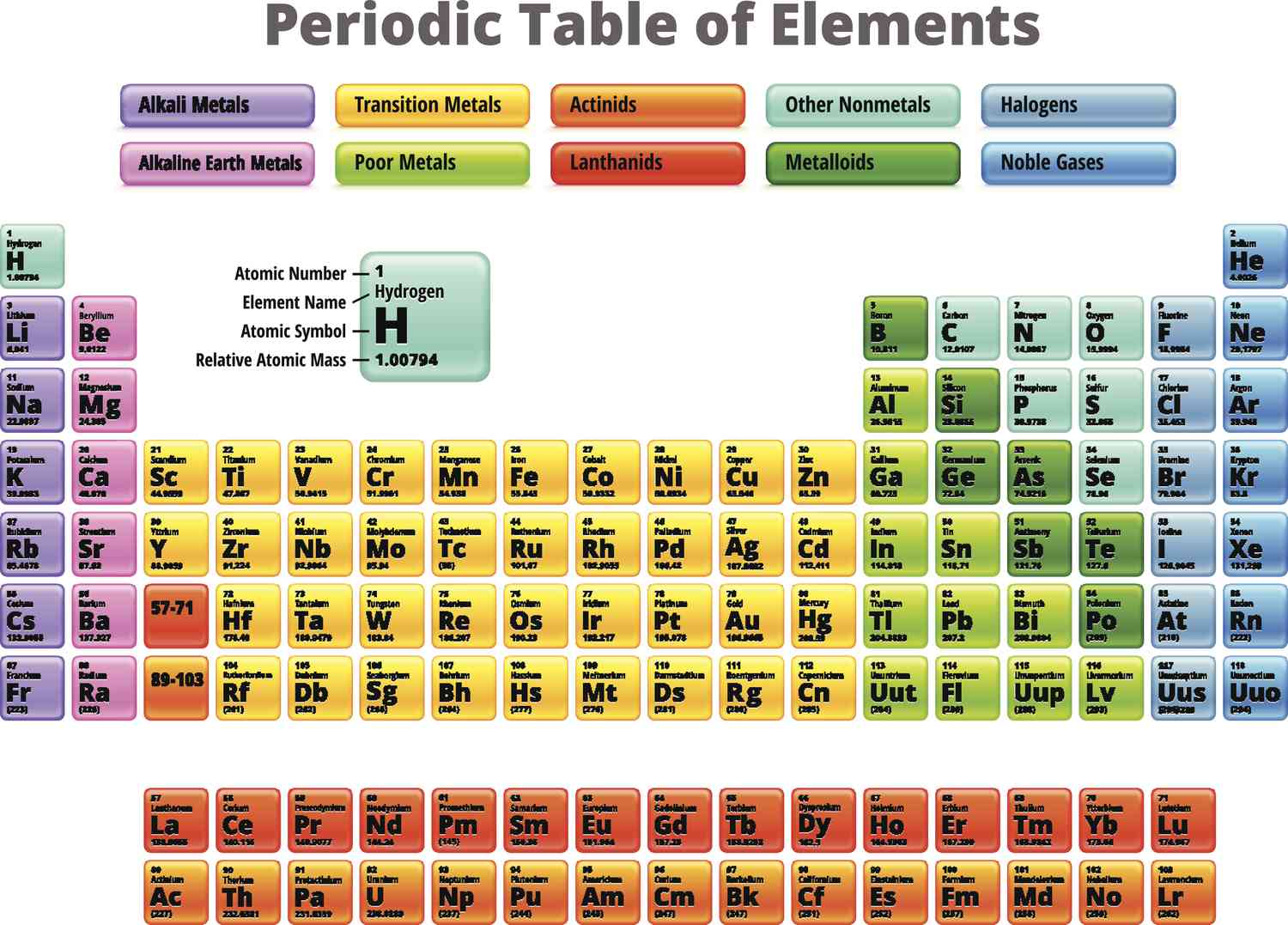
transition metals
good conductors, high luster
typically less reactive than alkali and alkaline-earth metals
some so nonreactive they do not easily form compounds & exist as free elements
groups 3-12
typically less reactive than alkali and alkaline-earth metals
some so nonreactive they do not easily form compounds & exist as free elements
groups 3-12
15
New cards
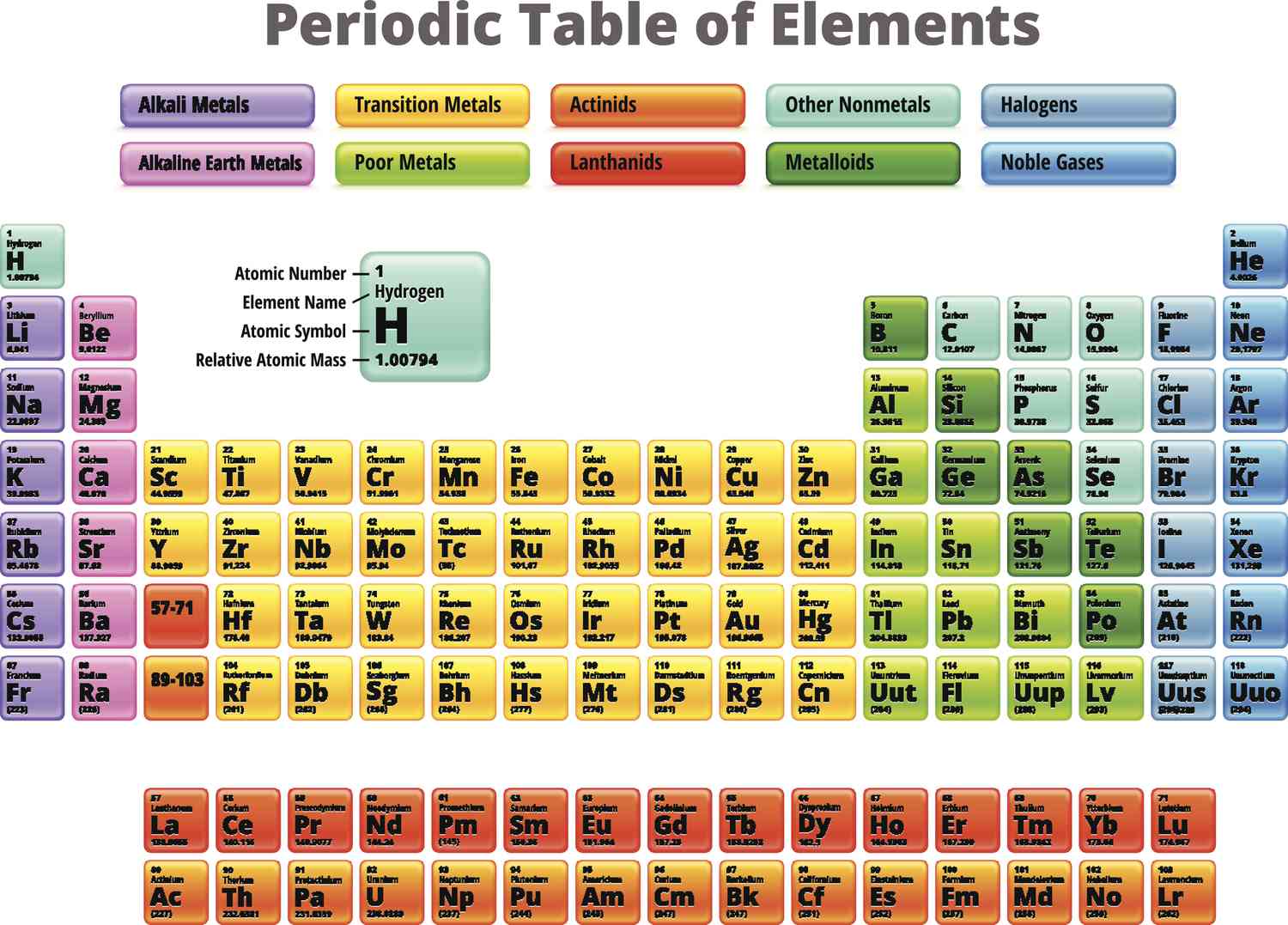
gold, palladium, platinum
What are the least reactive transition metals?
16
New cards
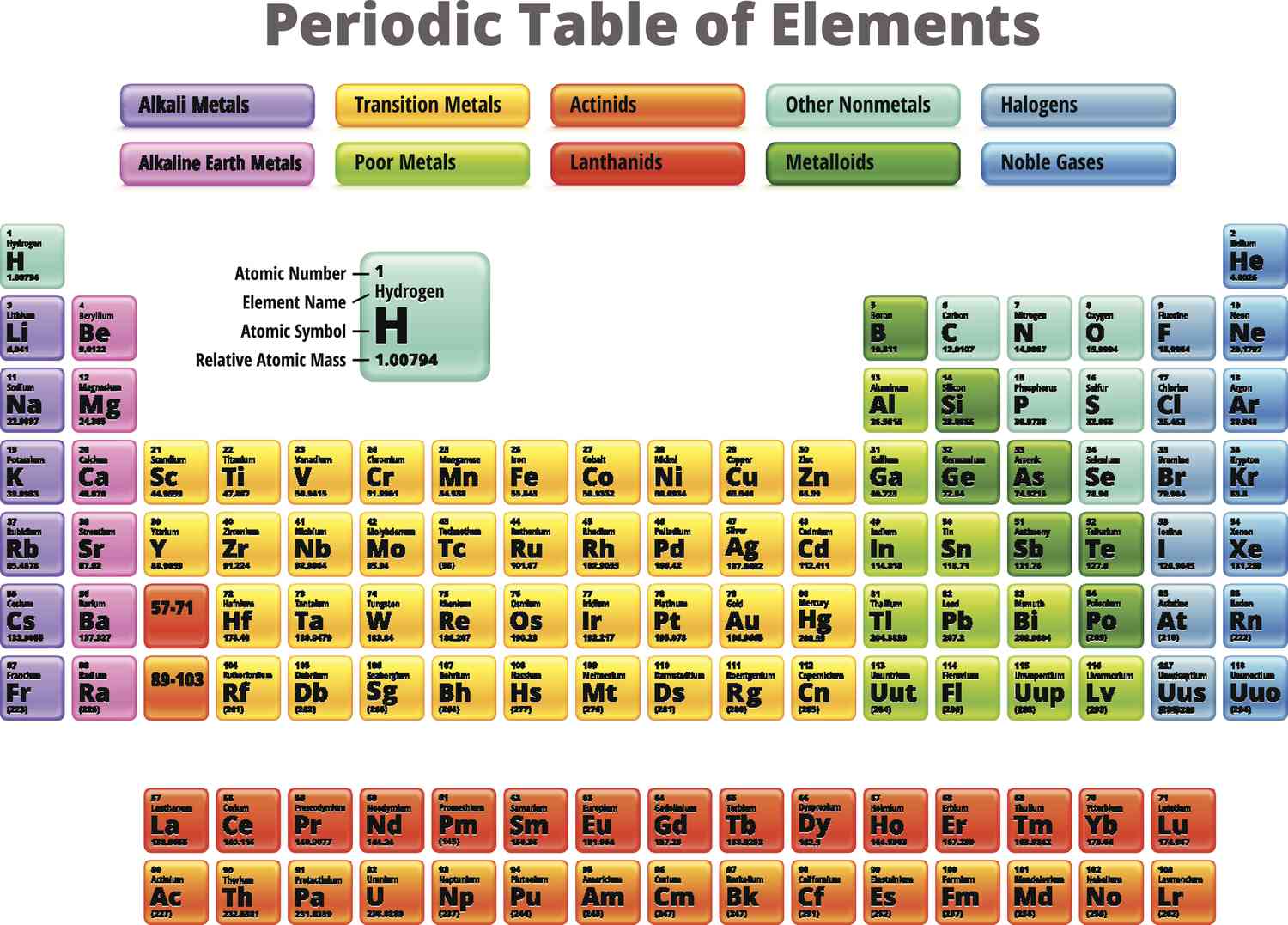
groups 13-18
what groups are the p-block elements?
17
New cards
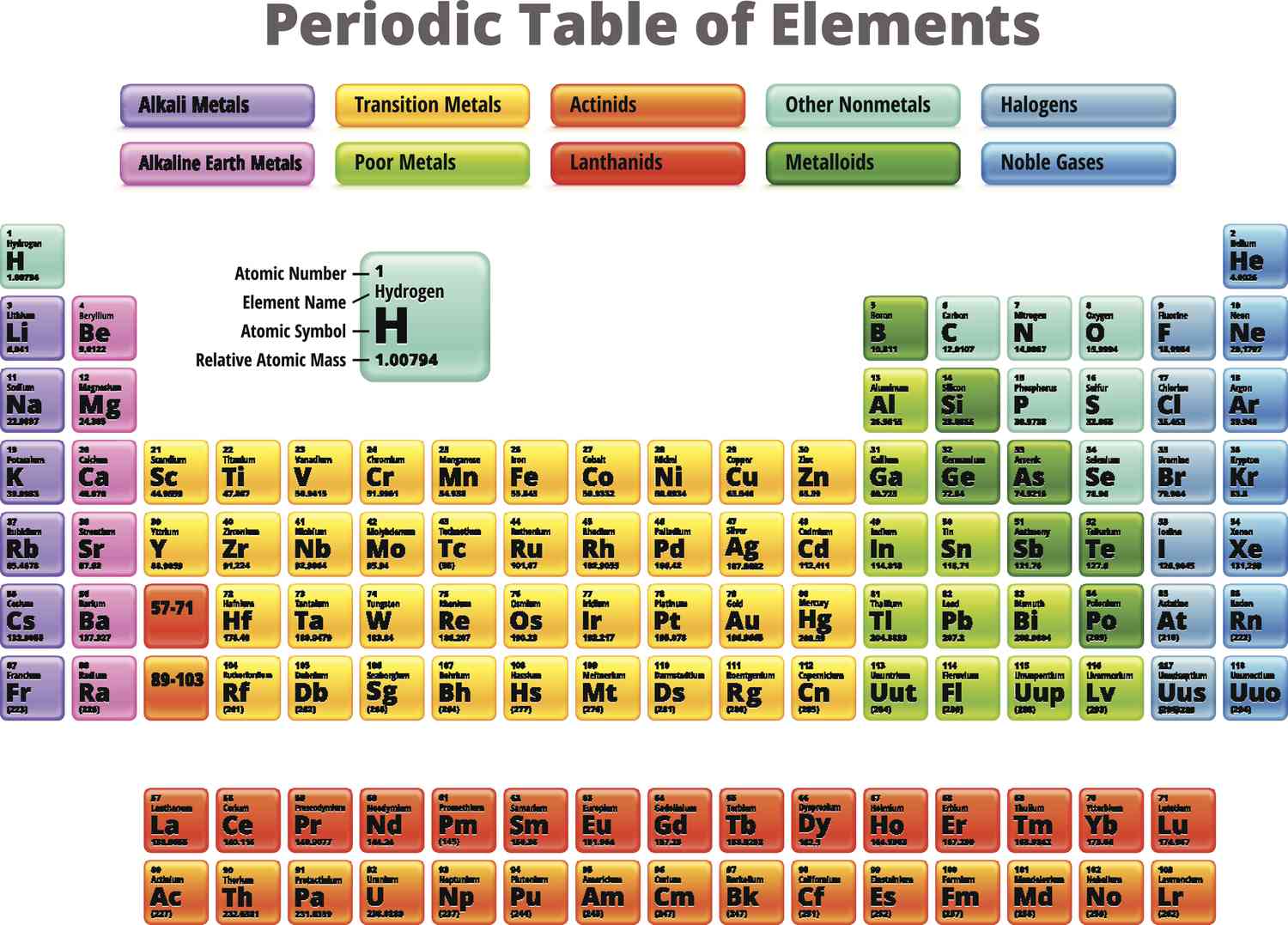
main group elements
made up by the s-block and p-block elements
properties vary greatly
properties vary greatly
18
New cards
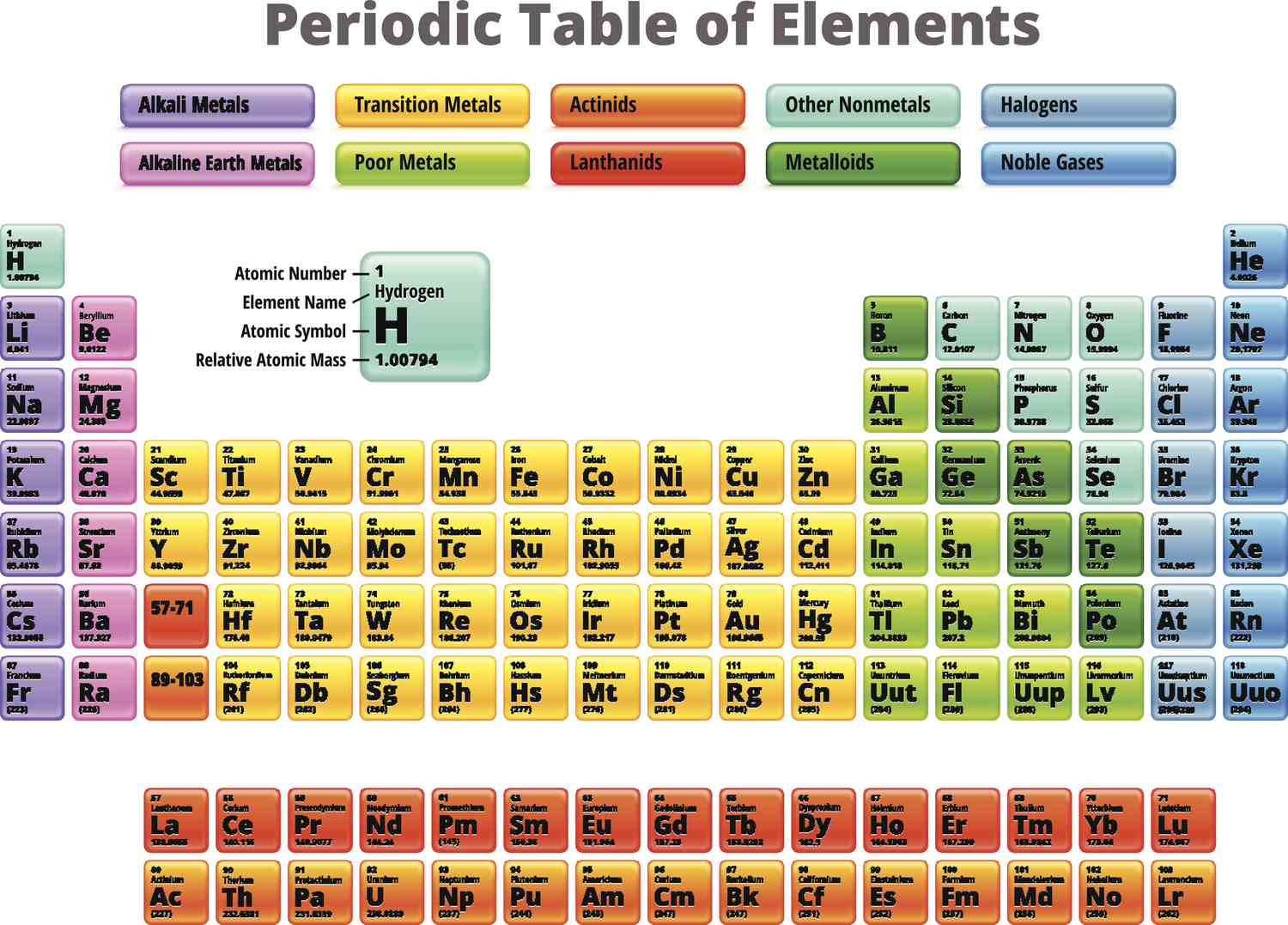
nonmetals
What kind of metals does the right side of the p-block have?
19
New cards
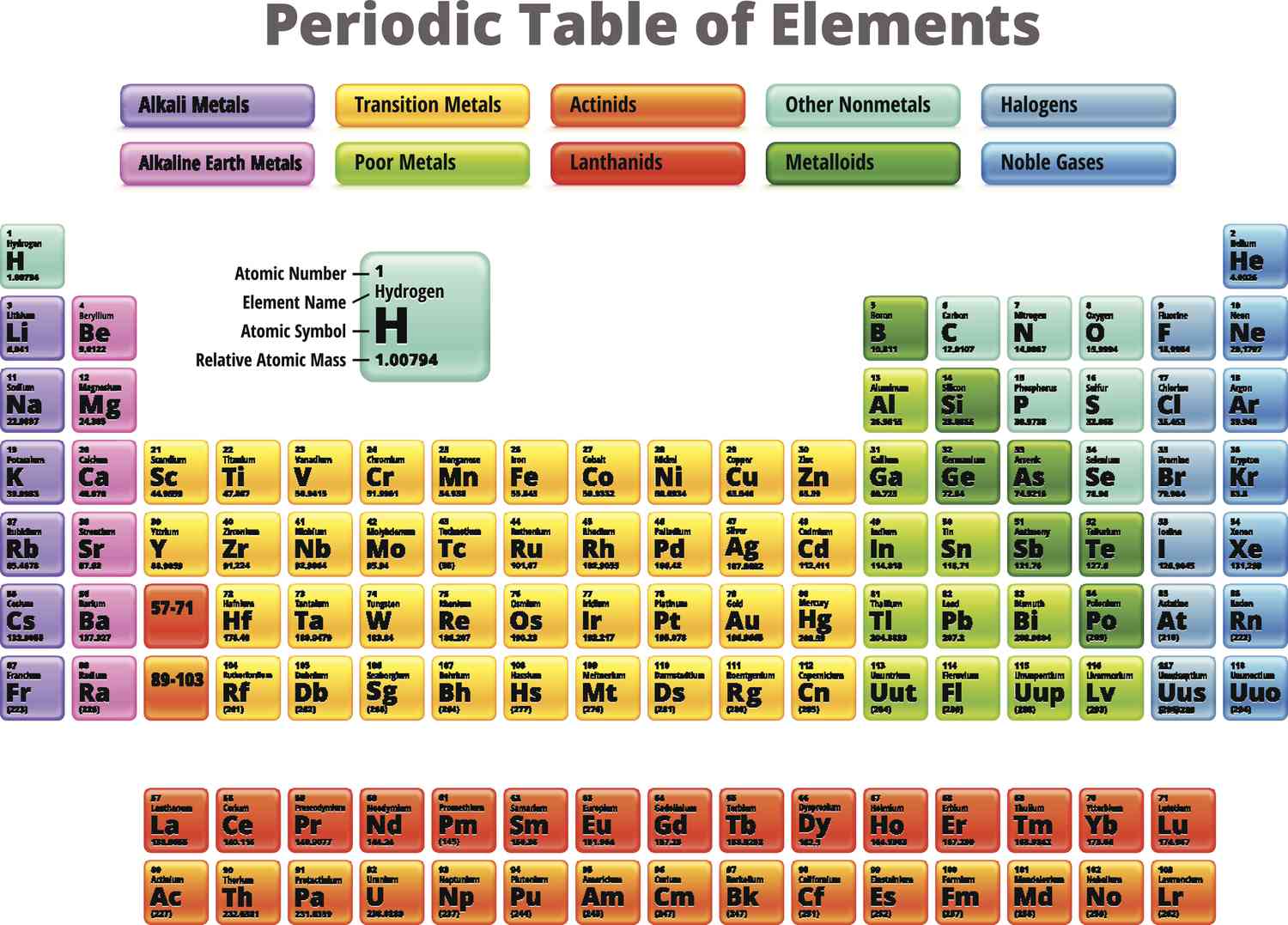
metals
What kind of metals does the left side and bottom of the p-block have?
20
New cards
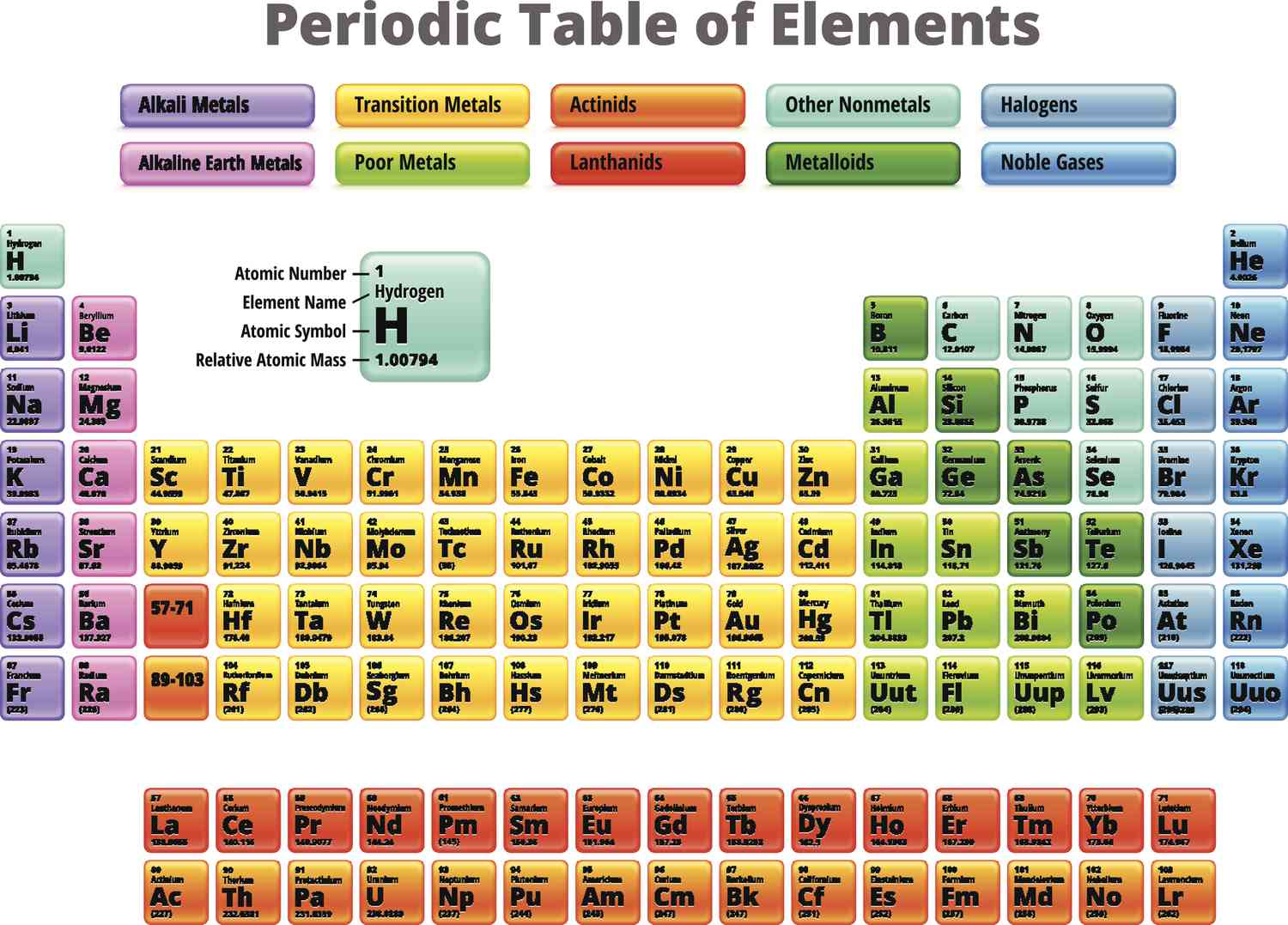
metalloids
boron, silicon, germanium, arsenic, antimony, tellurium
boron, silicon, germanium, arsenic, antimony, tellurium
What kind of 6 metals in the middle of the p-block called? What are they?
21
New cards
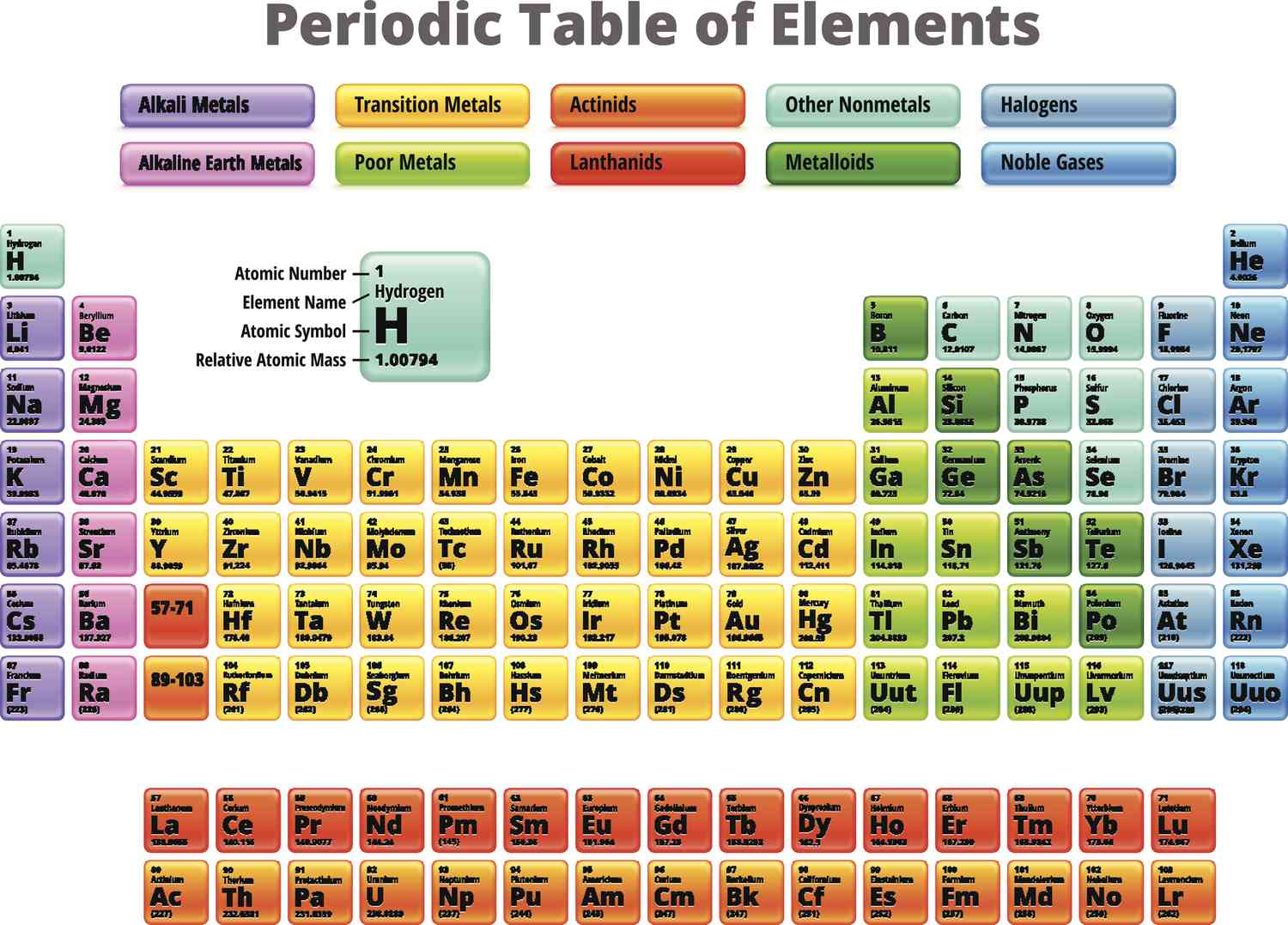
halogens
What is group 17 called?
22
New cards
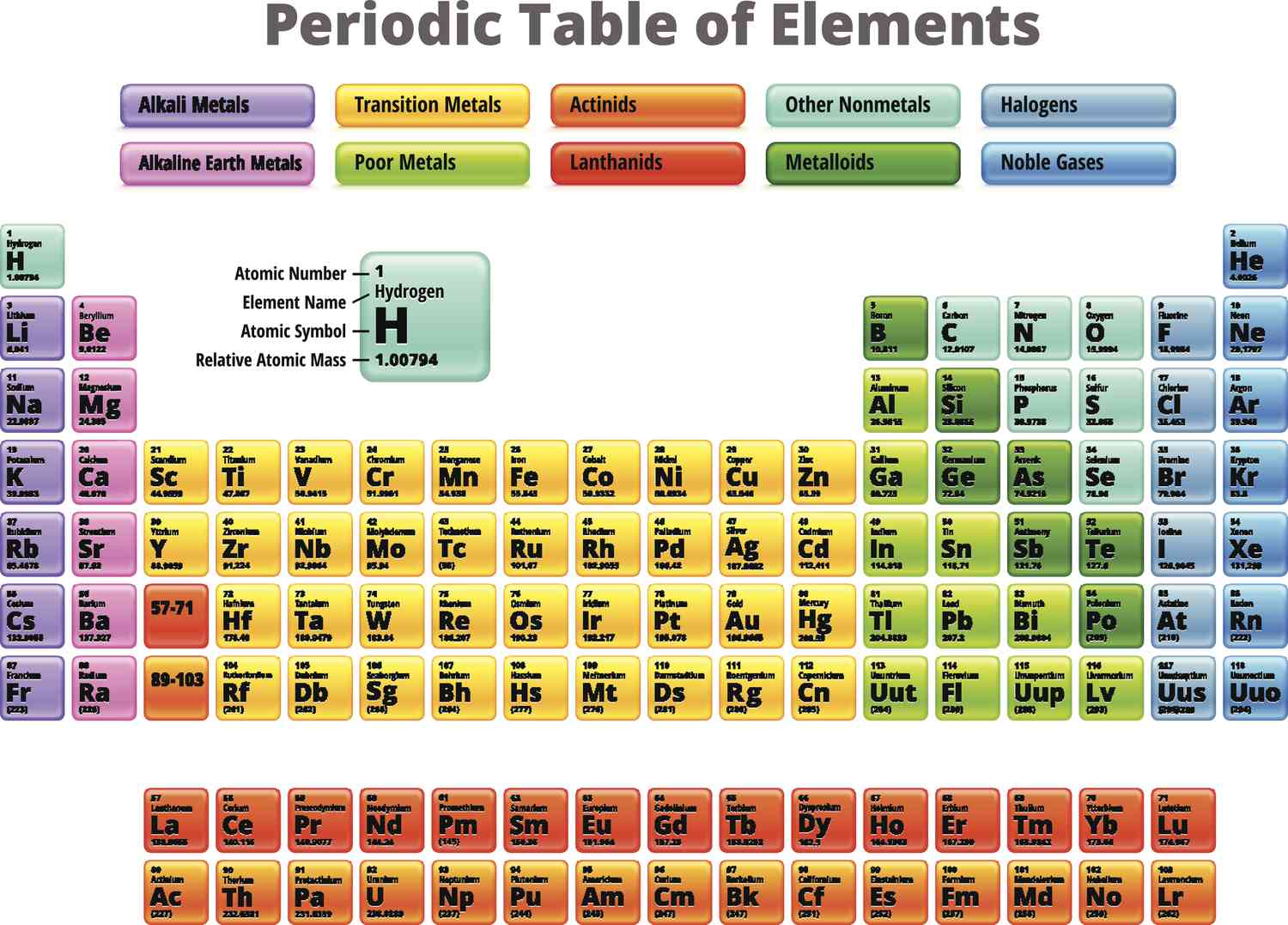
halogens
most reactive metals
react very vigorously with most metals to form salts
react very vigorously with most metals to form salts
23
New cards
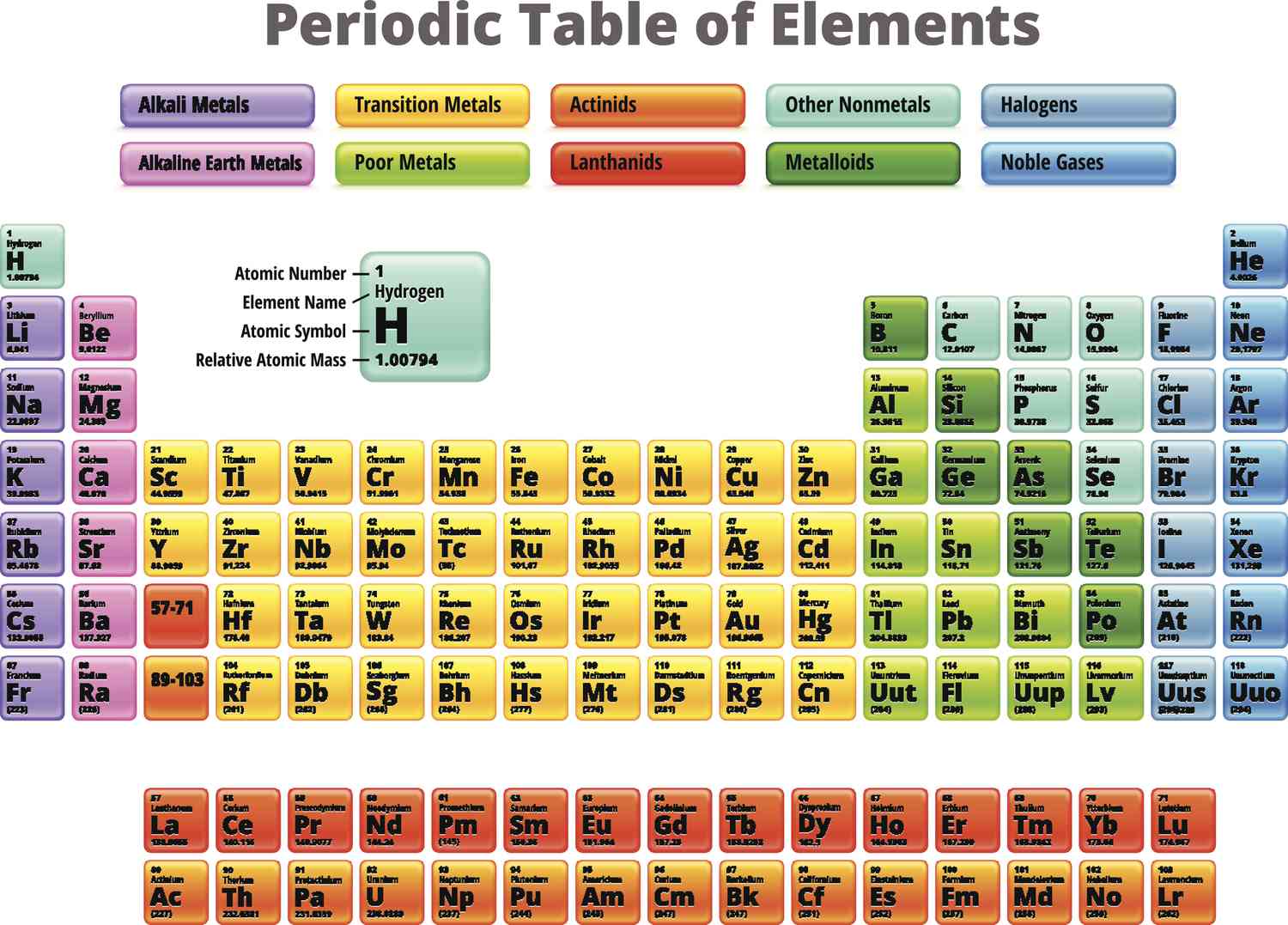
metalloids
semiconducting elements
located in the p-block between metals and nonmetals
brittle solids w/ properties of both metals and nonmetals
located in the p-block between metals and nonmetals
brittle solids w/ properties of both metals and nonmetals
24
New cards
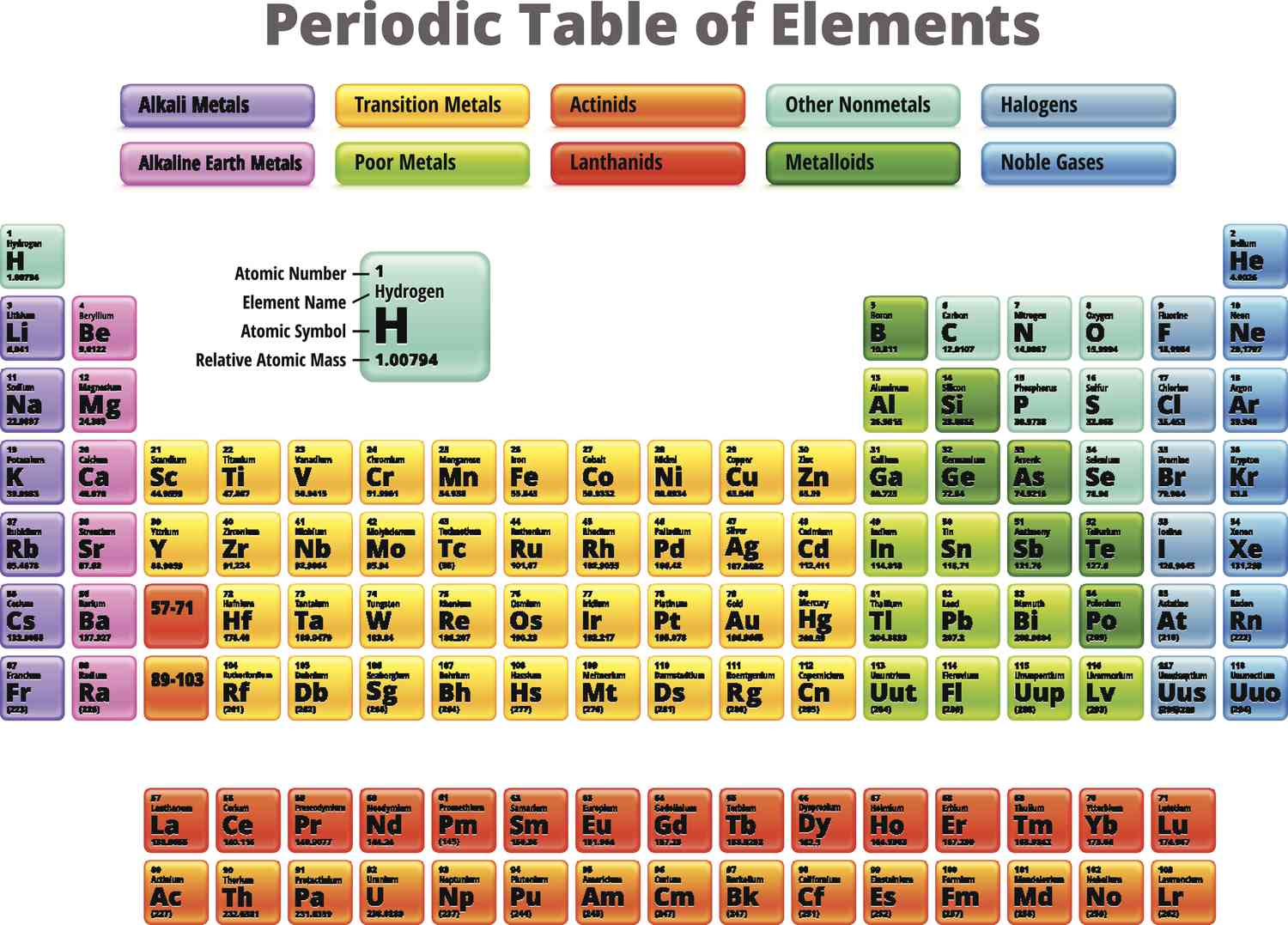
metals of the p-block
harder and denser than the s-block metals, but softer than the d-block metals
25
New cards
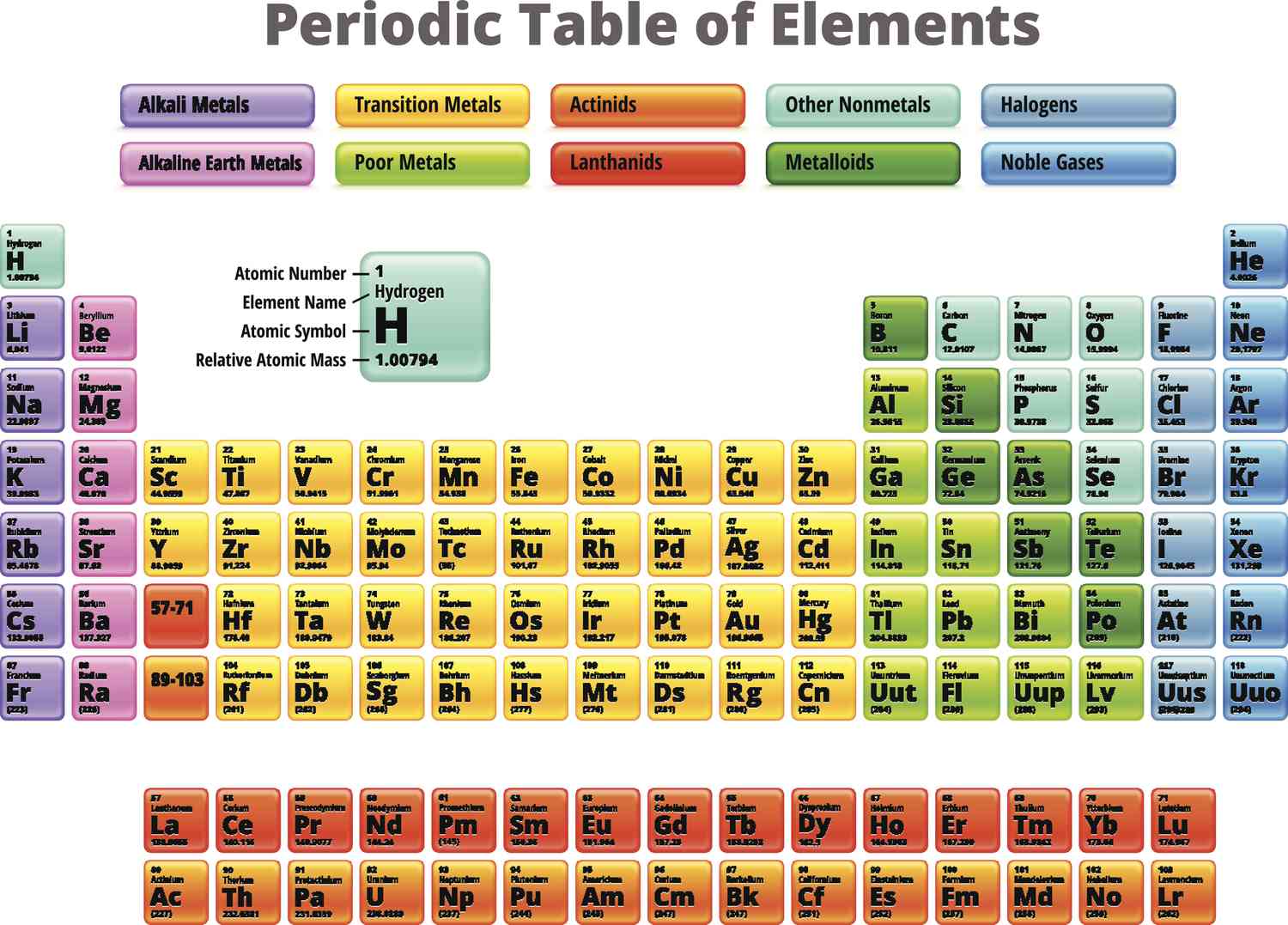
lanthanides and actinides
What does the f-block consist of?
26
New cards
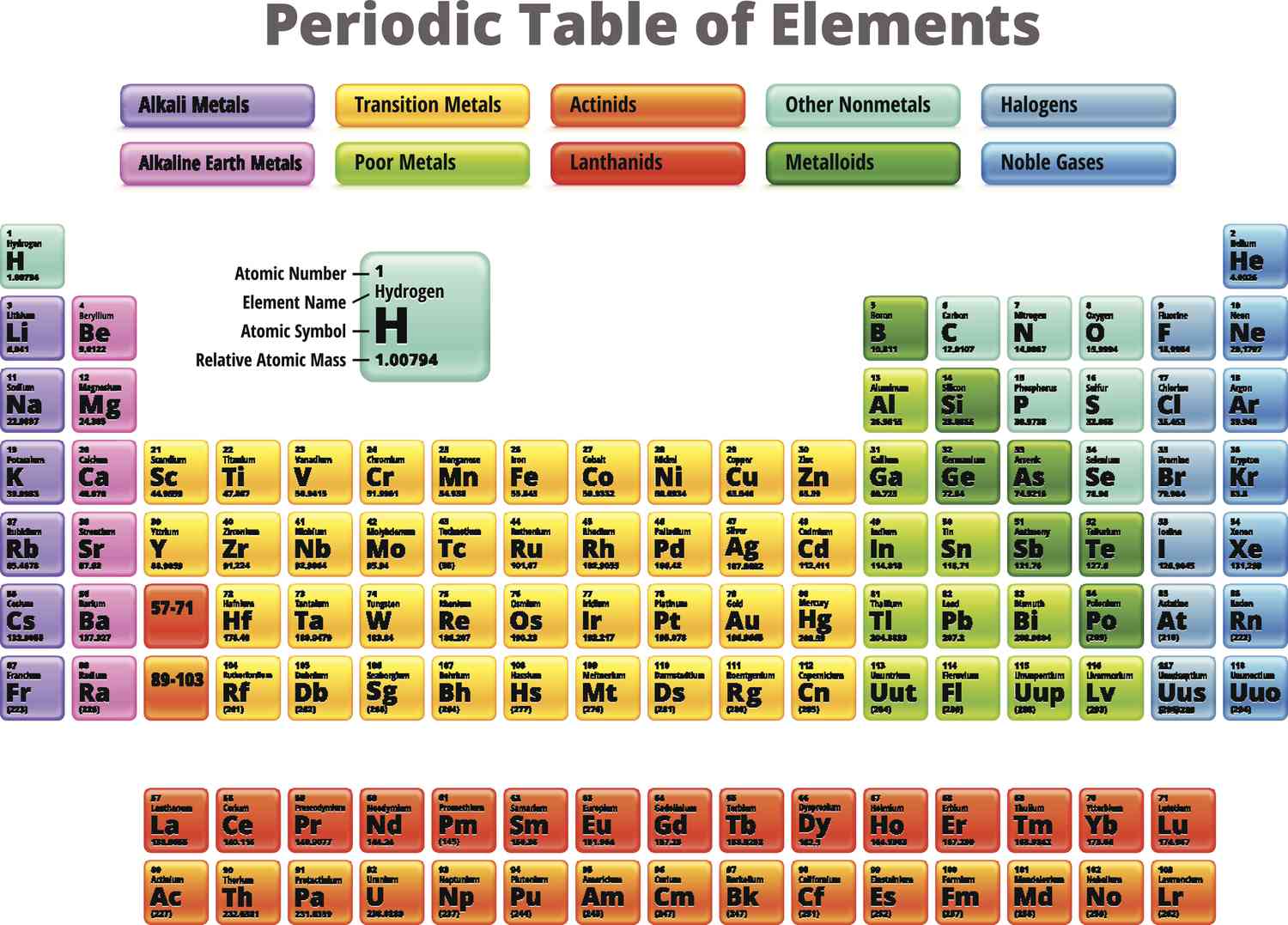
lanthanides
shiny and similar to group 2 reactivity
27
New cards
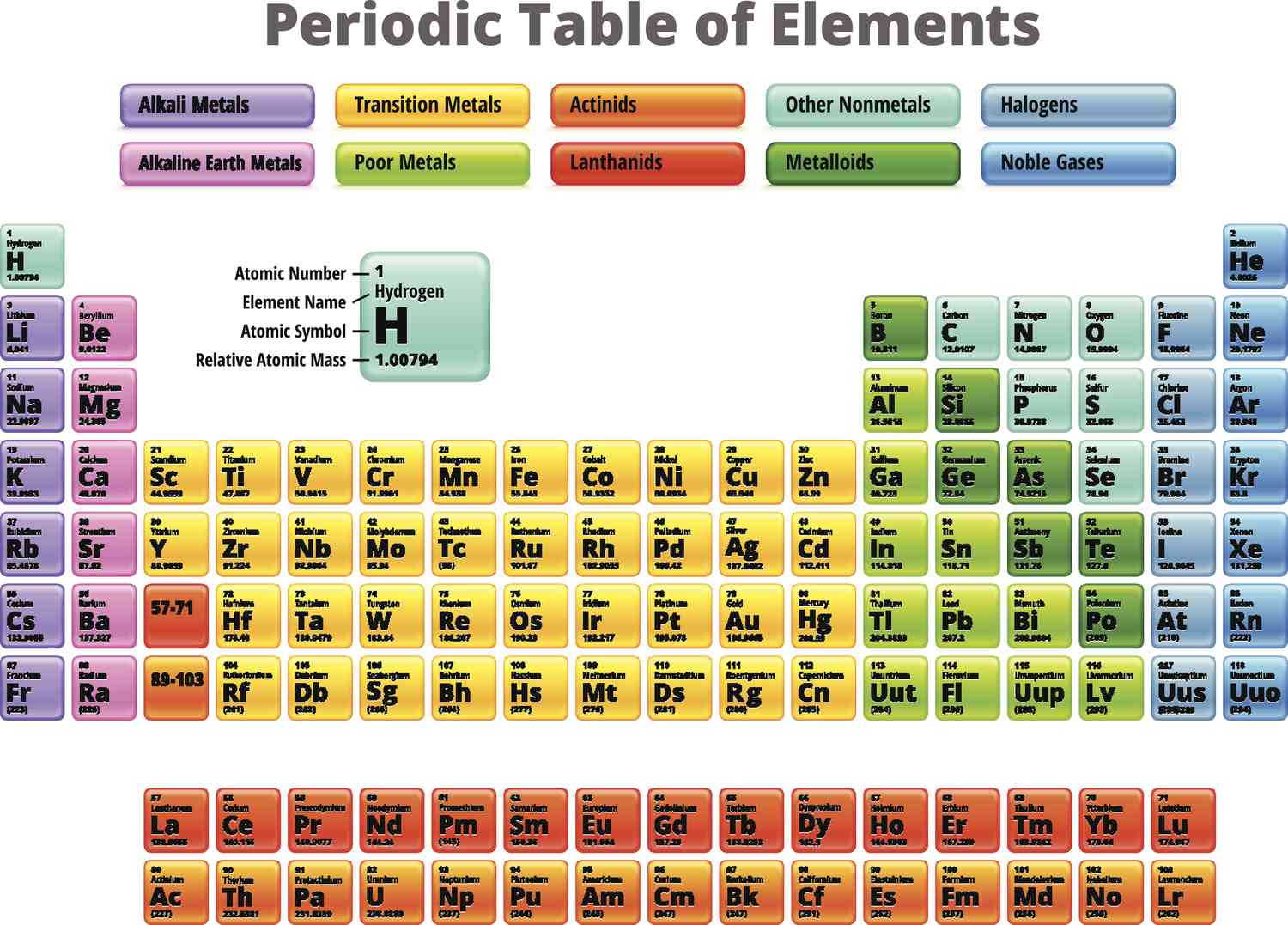
actinides
radioactive and only 1st four found naturally on earth, the rest made in the lab