Group 7 The Halogens and group 2 The Alkaline metals
1/68
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
69 Terms
What is the electron configuration of the elements in group 2 of the periodic table
Each of these elements have 2 electrons in an outermost s orbtial which is increasing more distant from the Nuecleus
Be Mg Ca Sr Ba
[He] 2s2 [Ne] 3s2 [Ar]4s2 [Ar]5s2 [Xe]6s2
What is the atomic radius trend for group 2
There is an increase in atomic radius down group 2 as the outermost electrons are in a principal quantum shell that is increasingly more distant from the nucleus
What is the trend in ionic radius down the group
Same reason as atomic radius. The ions are much smaller than their respective atoms due to the outer electrons being in an energy level closer to the nucleus so less shielding and greater attraction from the nucleus to the outer e-
What is the trend in ionisation energy as you go down group 2?
As you go down group 2 the 1st ionisation energy decreases. As outer electron is lost from an energy level further from nucleus. So greater shielding and weaker attraction from nucleus to outer e-
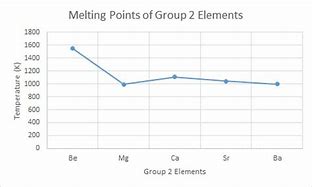
Trend in melting point of group 2
The melting points decrease down the group. Discontinually at Ca due to change from hexagonal to face centred cubic packing. The reason why it decreases is because the size of the ions increase down the group and the ions become increasingly less able to attract the deloclaised electrons, there is also greater shielding.
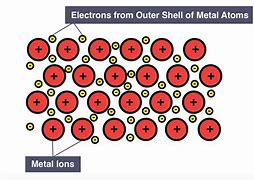
What is the general structureof a metal
They consist of cations in fixed positions in a lattice with the outside electrons delocalised and free to move. The metallid bond is the force of attraction between the positively charged ions for the delocalised electrons.
Observation and equation of mg reacting with water
Observation: very slow fizzing, mg sparingly dissolves, very fast reaction and white solid produced.
Equation: Mg +2H2O(l) → Mg(OH)2 + H2 or Mg + H2O(g) → MgO + H2
Observation and Equation for Calcium reacting with water
Observation : fast production of bubbles, fully dissolves. (If number in front of letter (little) if behind (big) )
Equation: Ca + 2H2O → Ca(OH)2 +H2
Observation and Equation of reacting strontium with water
Observation: Very fast production of bubbles, fully dissolves
Equation: Sr + 2H2O → Sr(OH)2 +H2
Observation and Eqaution for reacting barium with water
Observation: fastest production of bubbles fully dissolves.
Equation: Ba + 2H2O → Ba(OH)2 +H2
Trend of reactivity of group 2 metals and explanation of trend
Ba> Sr> Ca> Mg
Most Least (Opposite to trend in first ionisation energy)
Why? → A metal atom gets larger, outer electrons are held further from the nucleus, greater shielding, weaker attraction from nucleus to outer electron, outer electron is more easily lost.
Why is beryllium unique with the chemical reactivity regarding water
It does not react with cold water or steam
What is the trend for group 2 metals of their reactivity with water
It dcreases down the group
Magnesium reaction with cold water and steam (w EQUATION)
It reacts slowly with cold water but quickly with steam
Equations: Cold water: Mg +2H2O (l) → Mg (OH)2 +H2
Steam: Mg + H2O (g) → MgO + H2
Reaction of Calcium, strontium and barium down the group.
They react with cold water with increasing vigor as they go down the group
Trend in solubility in hydroxides
Increases as you go down the group
Ba(OH)2 > Sr(OH)2 > Ca(OH)2 > Mg(OH)2
Mg(OH)2 → Slightly soluble Ca(OH)2 → Slightly soluble
Ba(OH)2 → Soluble Sr(OH)2 → Soluble
Equation for precipitates formed by group 2 hydroxides
Mg2+ + 2OH- → Mg(OH)2
Ca2+ + 2OH- → Ca(OH)2
Why does Iodine sublime?
Because as a solid and liquid the van der waals forces between molecules are very similar in both states.
Solubility trend for group 2 sulphates
Decreases down the group
BaSO4 < SrSO4 < CaSO4 < MgSO4
MgSO4 → Soluble CaSO4 → Soluble
SrSO4 → Slightly Soluble BaSO4 → Insoluble
Ionic equation for formation of any precipitate.
Sr2+ + SO43- → Sr SO4
Ba2+ + SO42- → BaSO4
Observation and Equation for the reaction between barium chloride and sulphuric acid
Ba 2+ +SO42- → BaSO4
Observation white precipitate.
Observation and equation of Barium chloride and sodium hydroxide
No reaction
Observation and equation for magnesium chloride and sodium hydroxide
Mg2+ +2OH- → Mg(OH)2
Observation: white precipitate
Observation of calcium chloride and equation
Ca2+ +2OH- → Ca(OH)2
Small amount of white ppt
Observation and equation of reaction between calcium chlordie and sulphuric acid
No reaction
Observation and Equation of magnesium chloride and sulphuric acid
No reaction
Use of these compounds ( Mg(OH)2) Ca(OH)2 BaSO4)
Mg(OH)2, indigestion tablets/ antacid. Ca(OH)2 neutralising acidic soil. BaSO4, Barium meal as BaSO4 is insoluble
Group2 metal carbonates properties
Insoluble and react with acids
Equations for Group 2 metal carbonates and acids
MgCO3 + 2HNO3 → Mg(NO3)2 + H2O + CO2
CaCO3 + H2SO4 → CaSO4 +H2O +CO2
BaCO3 + 2HCl → BaCl2 +H2O +CO2
What do group 2 metal carbonates always produce with acid
all produce water and bubbles of CO2, to test for CO2 bubble through limewater and if it turns cloudy it is present.
How to extract titanium
Rutile titanium (IV) oxide (TiO2) is first converted to titanium (IV) chloride using carbon and chlorine
TiO2 +2Cl2 +2C → TiCl4 +2CO Cl2 = oxidising agent C= reducing agent
The TiCl4 is purified by fractional distillation before being reduced to Ti by Mg at 1000 degrees celsius in an argon atmosphere. TiCl4 +2Mg → MgCl2 + Ti
(Mg is reducing agent)
Removal of sulfur dioxide from flue gases
Burning fossil fuels produce SO2(g) due to sulphuric impurities. This is an ACIDIC GAS and is removed from flue gases with an ALKALI or BASE by WET SCRUBBING. A slurry is made by mixing calcium oxide (CaO) or calcium carbonate (CaCO3) with water and sparying on flue gas. A solid waste product calcium sulfate (IV) is formed
CaO + SO2 → CaSO3 (s)
or CaCO3 +SO2 → CaSO3(s) + CO2
How to complete a flame test
Dip nichrome wire in HCL ti clean it, then dip into known compound. Hold the loop in the clear blue port of the flame. Hold the loop in the clear blue port of the flame (Nichrome wire = High melting point)
Results of flame tests for positive ions
Sodium (Na+) = yellow
Calcium (Ca2+) = orange/red
Lithium (Li+) = Crimson
Potassium (K+) = lilac
Magnesium (Mg2+) = white
Barium (Ba2+) = green
Strontium (Sr2+) = red
Test for ammonium ions
Add dilute NaOH to unknown solution and warm gently
NH4+ + OH- → NH3 + H2O
Test the ammonia with damp red litmus paper will turn blue (alkaline)
Test for sulphate ions
Add nitric acid to remove any carbonate ions which would give a white ppt. Then add barium chloride (BaCl2) or Barium nitrate (Ba(NO3)2) solution. A white ppt of barium sulfate is formed ( Ba2+ + SO42- → BaSO4)
Test for hydroxide ions
These are alkaline so use red litmus, a positive result will turn red litmus paper to blue
Test for Halide ions
1) add nitric acid (aq) to the solution being tested → removes any other ions that could give a precipitate with silver nitrate (aq) (CO32- +2H+ → CO3 + H2O)
2)add silver nitrate (aq) to the solution being tested → this produces a precipitate for Cl-, Br-, I-
3) Add ammonia (aq) (dilute and conc) to the precipitate (This is used to see if the precipitate re dissolves to help confirm their identity).
What is produced when Silber nitrate/ dilute NH3/ Concentrated NH3 is added to Fluoride ion
AgNO3= No reaction, Dilute NH3 = ———— Cocentrated NH3 = —————
What is produced when Silber nitrate/ dilute NH3/ Concentrated NH3 is added to chloride ion
AgNO3 = white precipitate of AgCl (Ag+ + Cl- → AgCl) Dilute NH3 = dissolves Concentrated NH3 = dissolves
What is produced when Silber nitrate/ dilute NH3/ Concentrated NH3 is added to bromide ion
AgNO3 = Crean ppt of AgBr (Ag+ + Br- → AgBr) Dilute NH3 = insoluble Concentrated NH3 = dissolves
What is produced when Silber nitrate/ dilute NH3/ Concentrated NH3 is added to iodide ion
AgNO3 = Yellow ppt (Ag+ + I- → AgI) Dilute NH3 = insoluble Concentrated NH3 = insoluble
Test for carbonates
Add nitric acid and a fizz of CO2 gas is produced (CO32- + 2H+ → CO2 + H2O)
Bubble gas through limewater and it will turn cloudy/milky (Ca(OH)2 + CO2 → CaCO3(s) +H2O)
What are 2 unique things about group 7 the halogens
They are non metals and exist as discrete diatomic molecules F2, Cl2, Br2, I2,
They only need 1 electron to achieve their full outer shell.
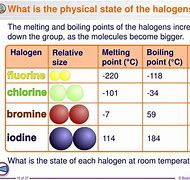
Trend in properties of group 7 (image) (states in flashcard)
Fluorine (gas)
Chlorine (gas)
Bromine (liquid)
Iodine (Solid)
What is the trend in atomic radii down grouo 7 and the reason
The atomic radii increase down the group as the outermost electrons are in a principal quantum shell increasingly more distant from the nucleus. Increasing atomic radius as outer shell electrons in an energy level further from the nucelus so weaker shielding and weaker attraction from the nucleus to outer shell electrons
What is the trend of ionic radius in group 7 halogens down the group and the reason
There is an increase in ionic size down the group. The ionic radius though is slightly larger than the corresponding atomic radius, the ion is slightly larger than its corresponding atom because there are more electrons in the same outer energy level; so they are held further from the nucleus.
Trend in melting and boiling points for group 7 halogens and the reason
All halogens are simple covalent, n on polar molecules. As the molecules get larger (Mr increases) the van der waals forces between the molecules get stronger. More energy required to break.
What is the trend in electronegativity down group 7 and why
As you go down group 7 the electronegativity decreases, this is because as the atomic radius increases down the group there is greater shielding and weaker attraction from the nucleus to bonding electrons.
What is the trend in the oxidising properties of the halogens and why?
As you go down the group the oxidising power of the halogens decreases. This is because when a halogen acts as an oxidising agent, it is itself reduced and gains an electron. As the size of the halogen atoms increases down the group , the incoming electron is held at a greater distance from the nucleus which, therefore has less of a hold over the incoming electron (F2 most powerful oxidising agent, I2 least powerful oxidising agent)
Process of the reduction of halogens (halogen acts an oxidsing agent)
X= halogen ½ X2 (g) + e- → X- (g)
Why specifically is fluorine such a strong oxidising agent
The weakness of the F-F bond means that fluorine is a particularly strong oxidising agent
Displacement reactions amongst halides and halogens
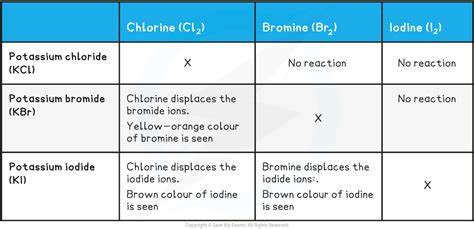
What is the reducing power of halide ions down the group
The reducing power increases, as fluorine is the most powerful oxidising agent of the group 7 elemetns, it follows that the fluoride ion is the last powerful of the halides as a reducing agent; as iodine is the least powerful oxidising agent, it also follows that the iodide ion is the most powerful halide reducing agent. This is because larger ions means greater shielding and more likely to lose an electron
process of oxidation of halide (halide is reducing agent)
X-(g) → ½ X2 (g) + e-
How do you differentiate between the different halide ions
Reactions with concentrated sulphuric acid can be used to distinguish between the halide ions. In the first instance, the hydrogen halides are displaced from their salts by the less volatile sulphuric acid. HF and HCL do not react further as they are not sufficiently powerful reducing agents to reduce the concentrated sulphuric acid.
Acid base reactions of sodium fluoride and concentrated sulphuric acid and sodium chloride and concentrated sulphuric acid
NaF + H2SO4 → NaHSO4 +HF
NaCl + H2SO4 → NaSO4 + HCL
HF and HCL are shown as steamy white fumes
Both reactions stop here, there is no redox as F- and Cl- are not strong enough reducing agents
Acid base and redox reaction of Potassium bromide and sulphuric acid
KBr + H2SO4 → KHSO4 + HBr (HBr white fumes)
Followed by redox as Br- is a strong enough reducing agent to reduce the concentrated sulphuric acid
Redox: 2Br- → Br2 + 2e- (Oxidation)
H2SO4 + 2e- + 2H+ → SO2+ 2H2O (reduction)
Overall: H2SO4 +2HBr → SO2 +Br2 + 2H2O
SO2 = Colorless pungent gas, Br2 = orange/brown fumes
Acid base and redox reaction a) between Potassium iodide and concentrated sulphuric acid
Initially acid base reaction KI + H2SO4 → KHSO4 + HI
HI = steamy white fumes
Then one or more of the following redox reactions occur:
a) 2I- → I2 + 2e- (oxidation)
(S = +6 → +4) H2SO4 + 2e- +2H+ → SO2 +2H2O reduction
overall H2SO4 + 2H+ + 2I- → SO2 + I2 + 2H2O
SO2 = colourless pungent gas I2 = purple vapour
redox reaction b) between Potassium iodide and concentrated sulphuric acid
2I- → I2 + 2e- (oxidation)
(S = + 6 → O) H2SO4 +6e- + 6H+ → S + 4H2O (reduction)
Overall: H2SO4 + 6HI → S + 3I2 + 4H2O
redox reaction c) between Potassium iodide and concentrated sulphuric acid
2I- → I2 +2e- (oxidation)
( S = + 6 → 2) H2SO4 +8e- + 8H+ → H2S +4H2O (reduction)
Overall: H2SO4 + 8HI → H2S + 4I2 + 4H2O
H2S = (Smell of rotten eggs) I2 = (purple vapour)
Oxidising and reducing agents and products for Acid base and redox reactions of halide ions
HBr/ HI = reducing agents
H2SO4 = oxidising agent
Br2/I2 = oxidation products
SO2/ S / H2S = reduction products
Why is the reasction between NaOH anc Cl2 a very important industrial process
It is responsible for the production of domestic bleach
Reaction with cold water wtih chlorine
= (reversible reaction symbol)
→ add NaOH - reacts with HCL/H+ Cold water: Cl2 (green) + H2O = HCl + HClO
Ox state: o 1 -2
add HCL ←
Reaction of hot water/ sunlight with chlorine
Cl2 + H2O → 2HCl + ½ O2 (oxidation of water)
What is the element that has been disproportionated in this reaction? (Oxidation and reduction occurs to one element)
Cl2
Chlorine reaction with sodium hydroxide
Cl2 + 2NaOH = NaCl + NaClO + H2O
NaCl = Sodium chloride, ox state of Cl = -1
NaClO = Sodium chlorate (I) ox state of Cl = +1
These are both disproportionation reactions
Water treatment explained
Add Cl2 to make water safer to drink/swim in since ClO- ions/ chlorate kills bacteria. Chlorine kills disease causing microrganisms and prevents the growth of algae and reinfection further down the supply.
Why is a low concentration of chlorine used in water treament
Chlorine is toxic, chlorine irritates the respiratory system and can cause chemical burns.