MSE 2001: 4.2 Amorphous Solids - Polymers
1/31
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
32 Terms
Monomers
A chain of repeat molecular units
High Molecular Weight
This type of molecular weight results in:
Greater elastic modulus
Greater toughness
Greater strength
Higher service temperature
Greater tensile strength
Homopolymer
A type of polymer structure that has the same repeating units
Copolymer
A type of polymer structure that has different repeating units
hang off
Side groups (aka pendant groups) on a polymer ____ ___ of the polymer backbone
Side group variations
rigidity, bulky like styrene
polar side groups, e.g. PVC (stronger van der waals + hydrogen bonding between chains, higher Tm)
length
longer, flexible side groups, decrease Tg
All of these are examples of…
Polymer properties
less dense, harder to pack, higher melting point, higher Tg, harder for chains to slide past each other
flexibility
density
strength
service temperature
These are examples of what affected by side group variations?
Atactic
A type of tacticity in which side groups are
randomly arranged
have less dense packing
are harder to crystallize
Isotactic
A type of tacticity in which side groups are
same-side arranged
are easier to crystallize
Syndiotactic
A type of tacticity in which side groups are
every-other arranged
are easier to crystallize
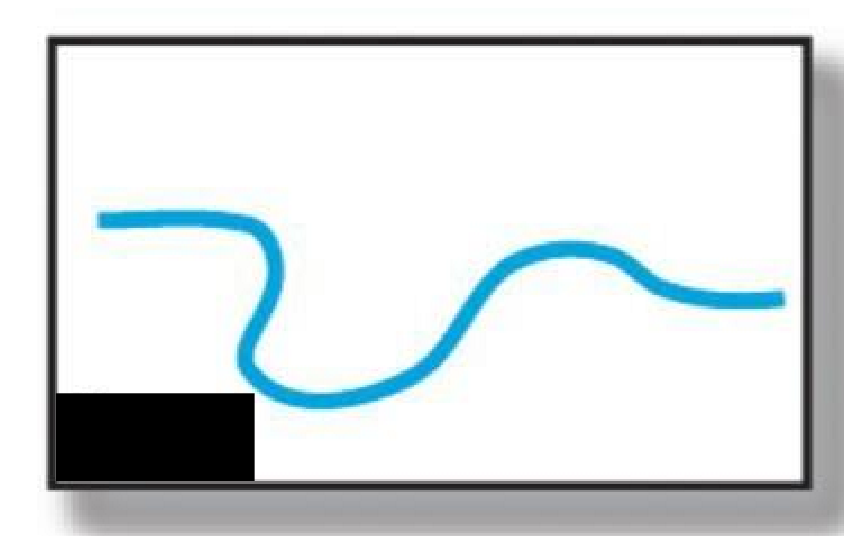
Linear
What type of polymer structure is this?
Comb
What type of polymer structure is this?
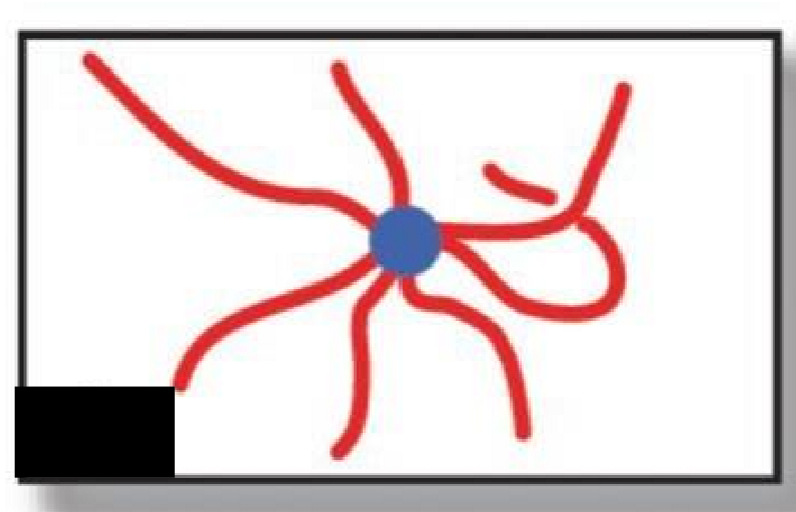
Star
What type of polymer structure is this?
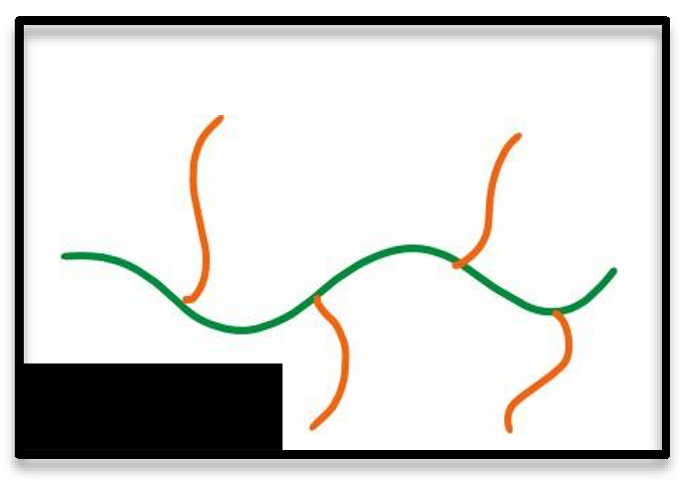
Grafted
What type of polymer structure is this?
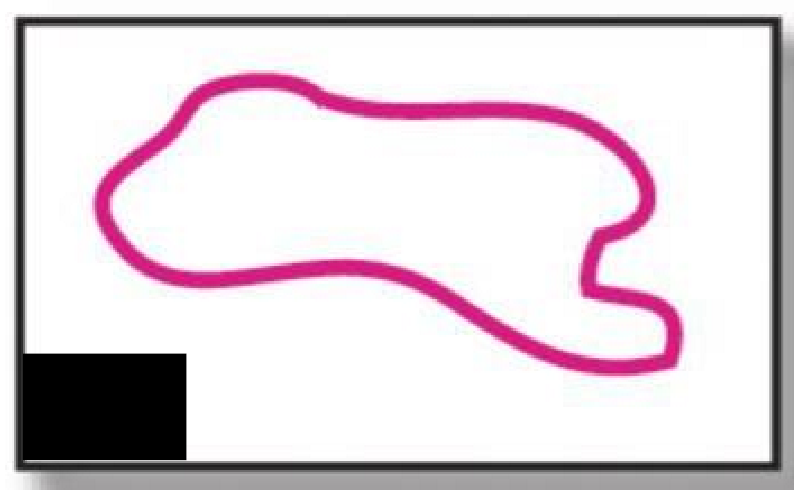
Ring
What type of polymer structure is this?
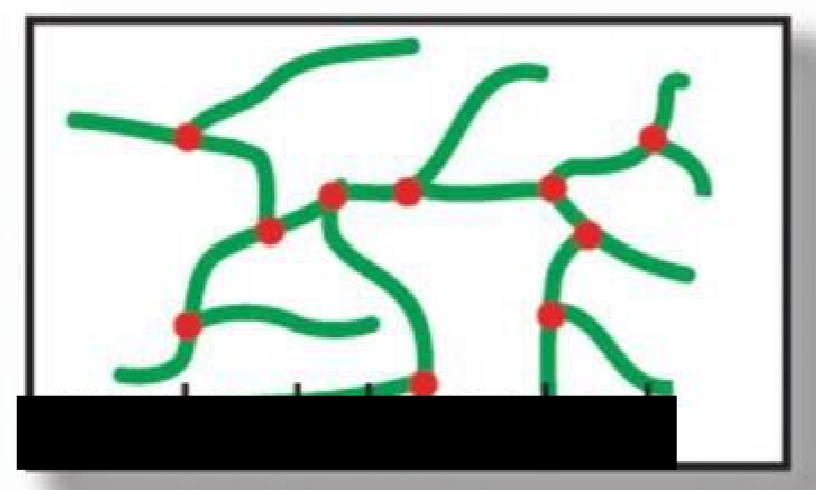
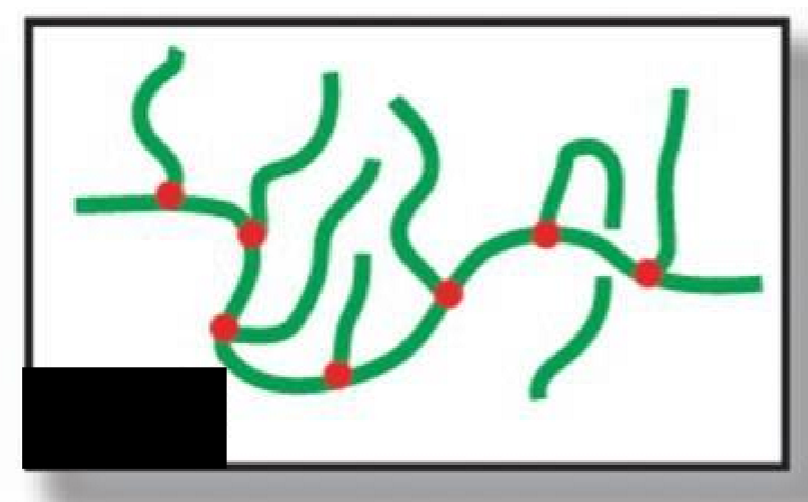
Randomly branched
What type of polymer structure is this?
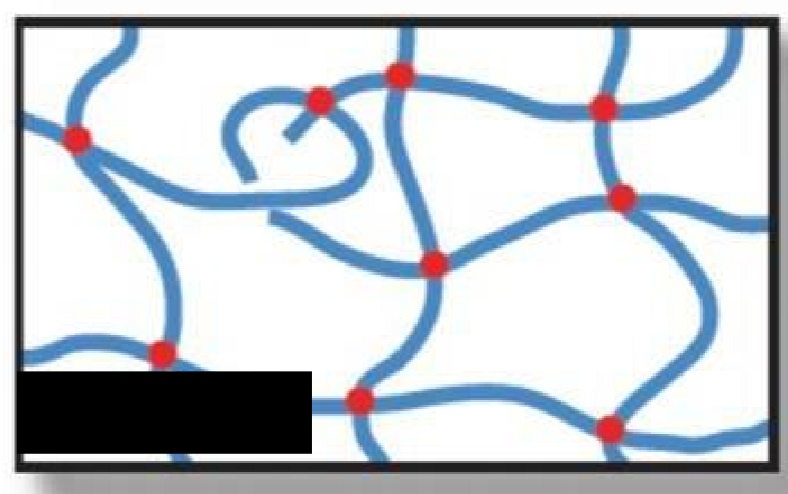
Network
What type of polymer structure is this?
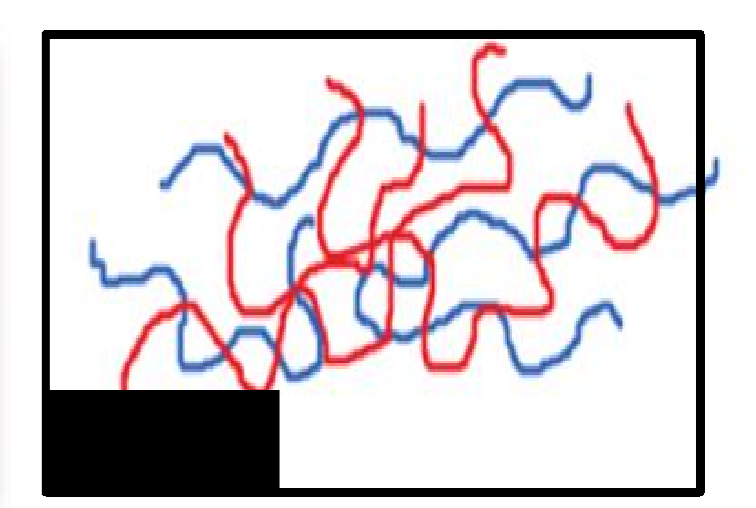
Blend
What type of polymer structure is this?
Blend
This type of polymer structure architecture is a physical mixture of two or more polymers
covalent
Chemical cross links involve ______ bonds between two polymer chains
Physical cross links involve ____ bonds between two polymer chains
increases
Increasing cross-linking (increases, decreases) strength and rigidity
Cross-linking methods
Catalysts (e.., vulcanization or photocuring)
network formers (monomers which can make 3+ bonds)
These are examples of…
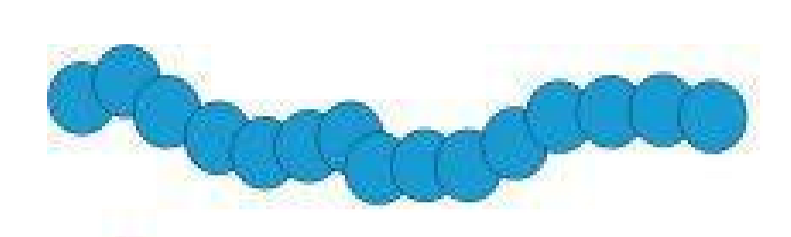
Homopolymer
What type of polymer is this?
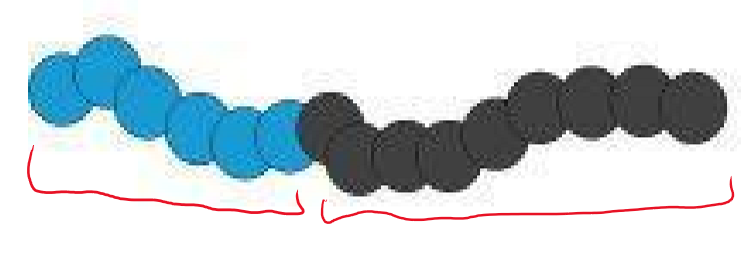
Block copolymer
What type of polymer is this?
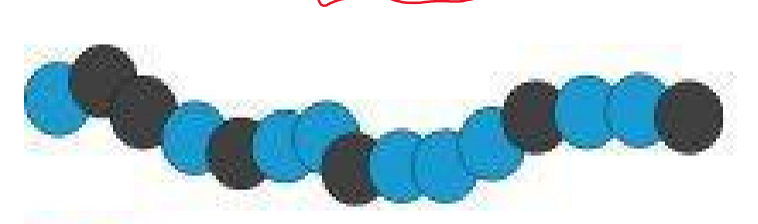
Statistical copolymer
What type of polymer is this?
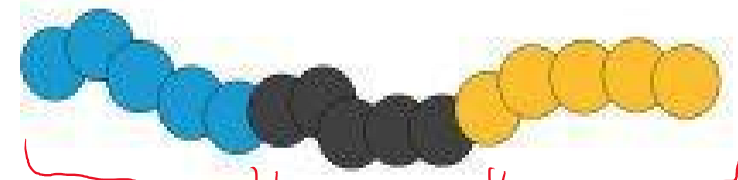
ABC triblock terpolymer
What type of polymer is this?
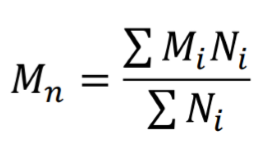
Number average molecular weight
What equation does this represent?
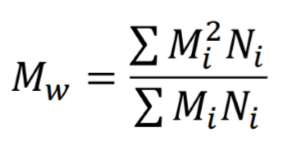
Weight average molecular weight
What equation does this represent?
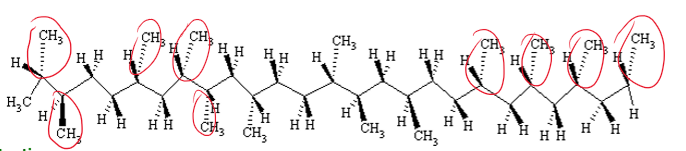
Atactic
What is the tacticity of this polymer?
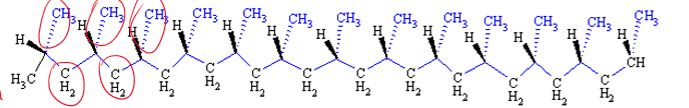
Isotactic
What is the tacticity of this polymer?
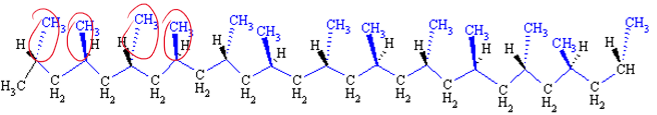
Syndiotactic
What is the tacticity of this polymer?