Group 7 elements
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
12 Terms
Group 7
Also called the halogens
Group 7: Physical States & Properties
Know the colours, physical states (at room temperature) and trends in physical properties of these elements
The Physical Properties of Halogens
Halogens: Group 7 non-metals that are poisonous and includes: Fluorine, Chlorine, Bromine, Iodine and Astatine.
Halogens are diatomic, meaning they form molecules of two atoms.
Colours and States at Room Temperature:
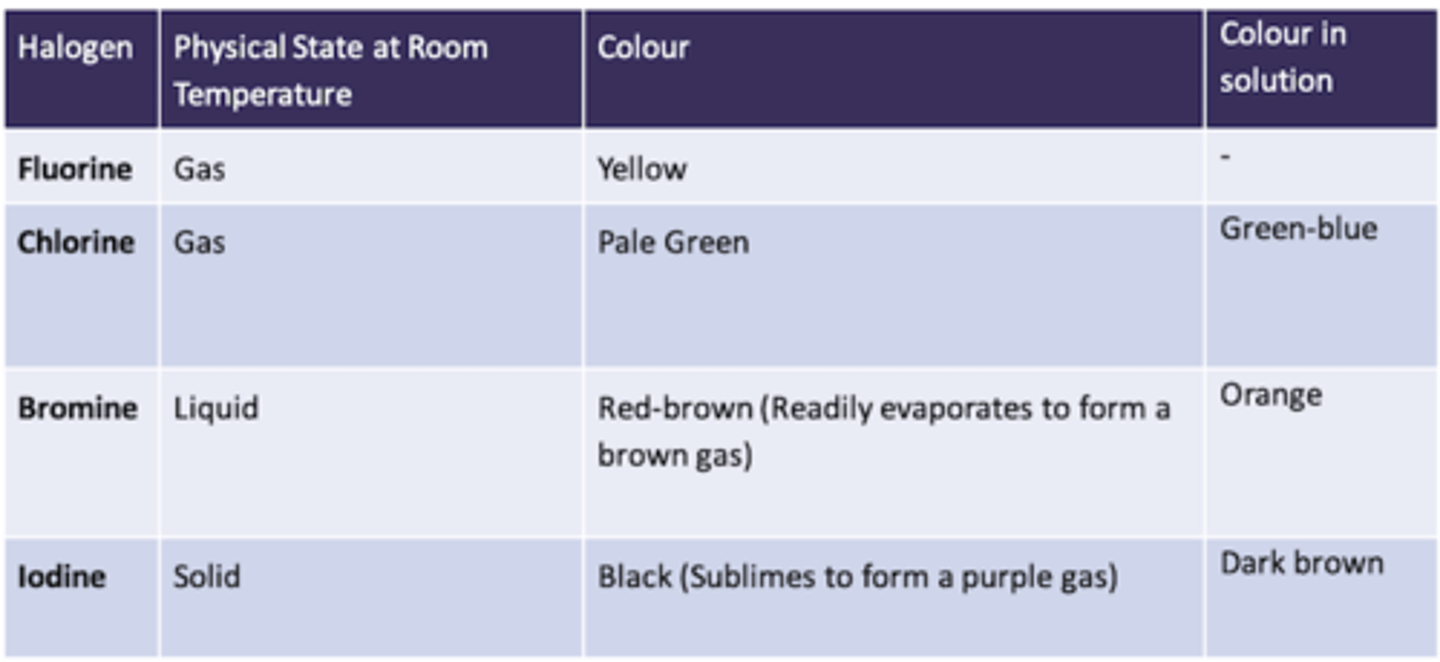
Trends in Physical Properties: melting point
Melting Point
The Melting and Boiling Points of Halogens increases as you go down the group
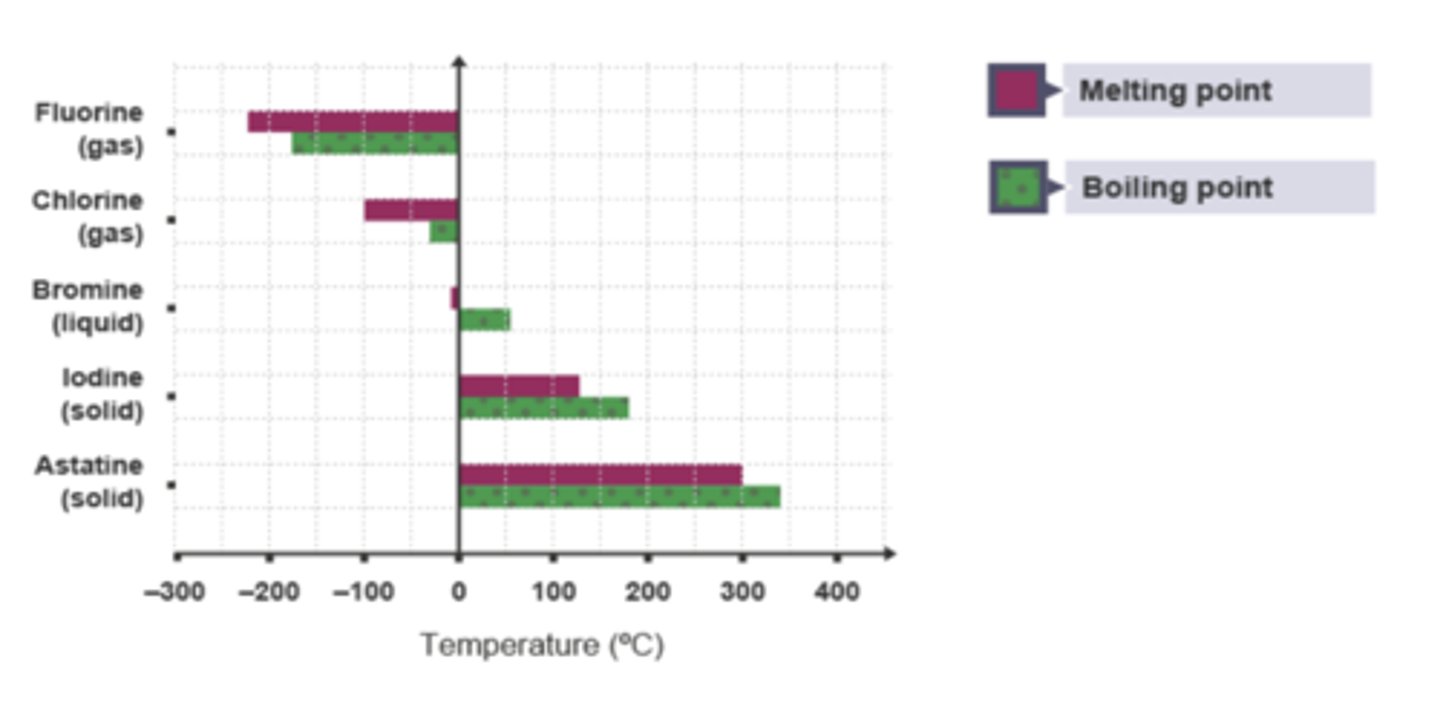
Trends in Physical Properties: at room temperature
At room temperature (20 °C), the physical states of the Halogens become more hard and set.
Chlorine is a gas, bromine is a liquid and iodine is a solid.
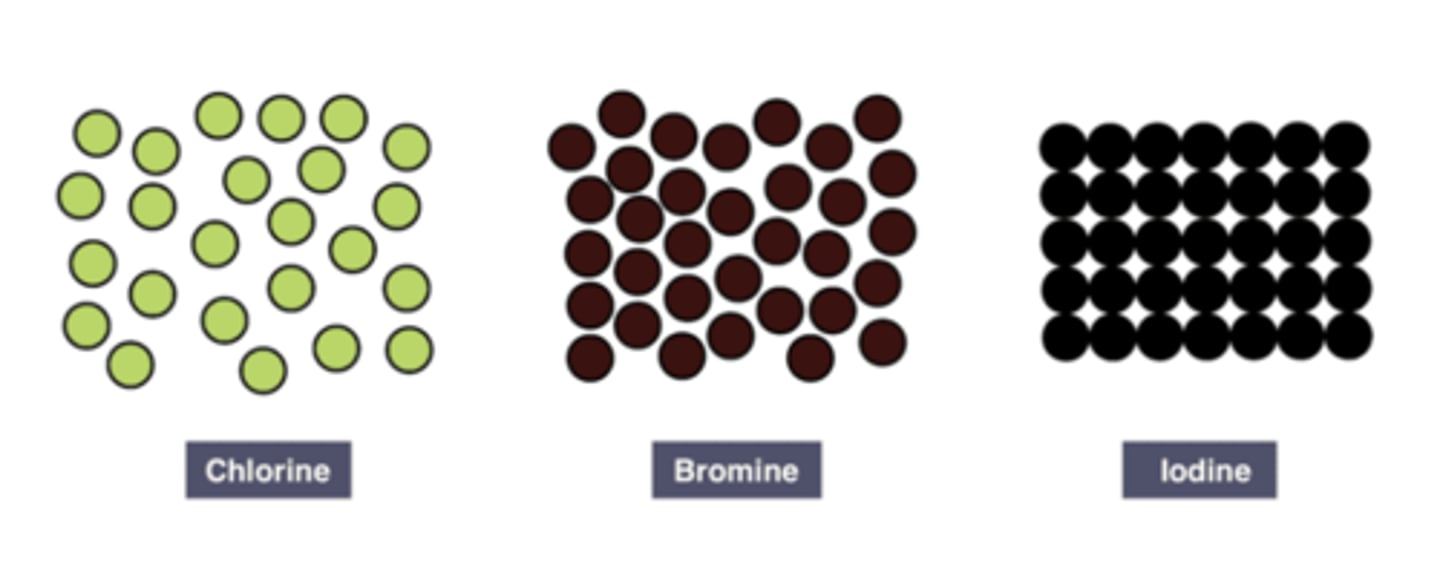
Colour of the halogen
The Halogens become darker as you go down the group.
Chlorine is pale green
bromine is red-brown
iodine is black.
Predicting the Properties of Halogens
Properties of other Halogens (Fluorine and Astatine):
Melting and Boiling Point
The melting and boiling point of Halogens increases as you go down the group
Fluorine is on the top of Group 7 so will have the lowest melting and boiling point
Astatine is on the bottom of Group 7 so will have the highest melting and boiling point
States
The states of Halogens become more hard and set as you go down the group
Fluorine is on the top of Group 7 so will be a Gas
Astatine is on the bottom of Group 7 so will be a Solid
Colour
The colour of Halogens become darker as you go down the group
Fluorine is on the top of Group 7 so the colour will be lighter: Yellow
Astatine is on the bottom of Group 7 so the colour will be darker: Black
Group 7: Reactivity
Understand how displacement reactions involving halogens and halides provide evidence for the trend in reactivity in Group 7
Halogens & Halides Displacement Reactions
Displacement Reaction: When a more reactive Halogen displaces a less reactive halogen for an aqueous solution of its halide.
Reactivity of Group 7 non-metals increases as you go up the group
Out of the 3 halogens, chlorine, bromine and Iodine, chlorine is the most reactive and iodine is the least reactive
Aqueous Solution Colour of Halogens:
Examples:
If you add chlorine solution to colourless potassium solution, the solution becomes orange as bromine is formed. Chlorine is above bromine in group 7 so is more reactive. Chlorine will therefore displace bromine from an aqueous solution of metal bromide:
Chlorine + Potassium Bromide Solution → Potassium Chloride Solution + Bromine
2KBr (aq) + Cl2 (aq) → 2KCl (aq) + Br2(aq)
Bromine is above Iodine in Group 7 so is more reactive. Bromine will therefore displace Iodine from an Aqueous
Solution of Metal Iodide:
Bromine + Magnesium Iodide Solution → Magnesium Bromide Solution + Iodine
Br2 (l) + 2MgI (aq) → 2MgBr (aq) + I2 (aq or s)

Electronic Configuration of Elements in Group 7
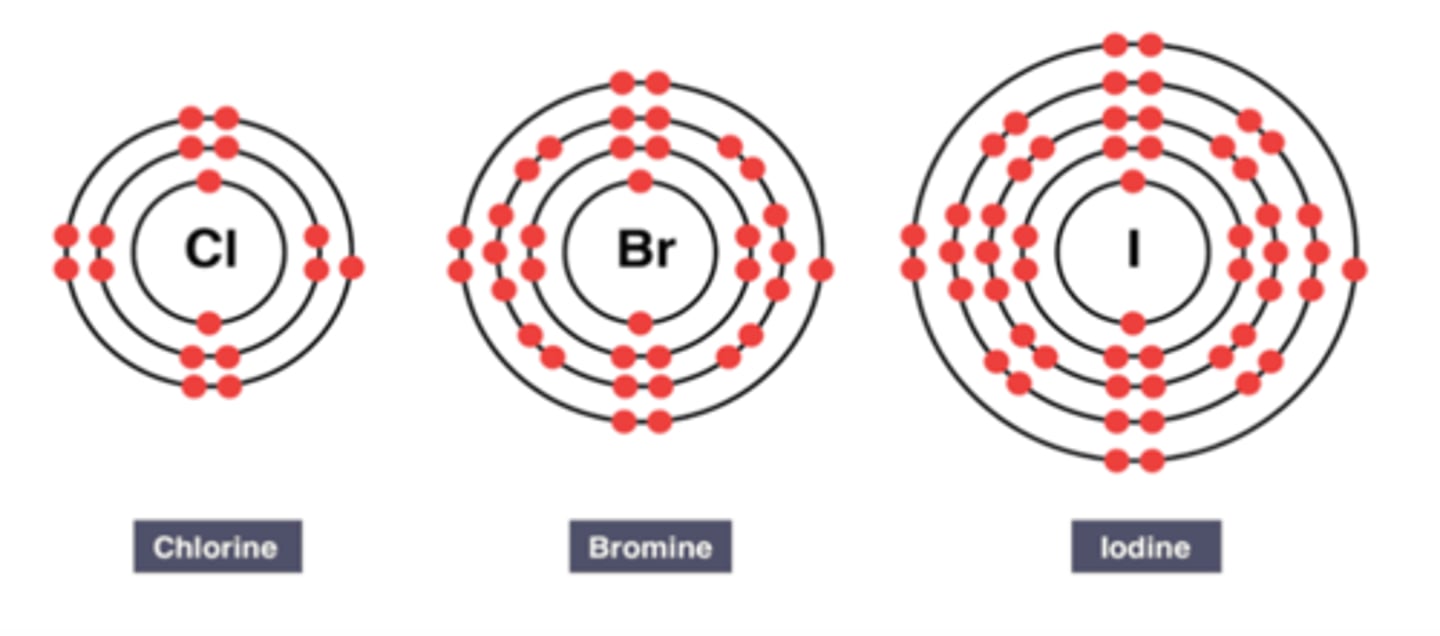
Trend in Reactivity of Group 7:
Reactivity of group 7 non-metals increases as you go up.
Each outer shell contains seven electrons and when group 7 metals react, they will need to gain one outer electron to get a full outer shell of electrons.
As you go up group 7, the number of shells of electrons decreases (period number decreases up the Periodic Table).
This means that the outer electrons are closer to the nucleus so there are stronger electrostatic forces of attraction that attracts the extra electron needed.
This allows an electron to be attracted more readily, making it more reactive as you go up the group.