BIO I (Neely) Flashcards - Practice Lecture Exam II
1/99
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
100 Terms
Ch.5 - What factors contribute to the fluidity of the plasma membrane?
bilayer fluidity/phospholipid movement, chemical properties, selectively permeable
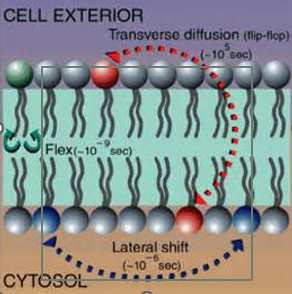
Ch.5 - How do phospholipids move in the bilayer and which movements are energetically favorable? Which movement is not energetically favorable?
Rotational movement - energetically favorable; “spinning”
Lateral movement - energetically favorable -10^7/sec; “Side to side” shift
Flip flop movement - energetically unfavorable; requires ATP & Flippase (catalytic enzyme); “ top to bottom / bottom to top” shift
Ch.5 - How does cholesterol contribute to the fluidity of the bilayer (either positively or negatively)?
Pros: provides support and structure to cell membrane, fluidity buffer, adapting the membrane's properties to the surrounding temperature
extremes temperatures decreases membrane fluidity (cold- membrane stiff and hot - membrane loose)
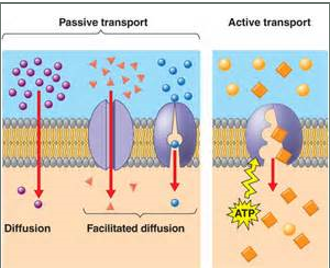
Ch.5 - What is the difference between simple diffusion, facilitated diffusion, and active transport? What does active transport require?
(Passive transport (PT) - no ATP needed):
simple diffusion (PT) - solute moves from an area of high concentration to an area of low concentration
Facilitated diffusion (PT) - a transport protein provides a passageway for a solute to move down the concentration gradient and diffuse across a membrane
Active Transport - requires ATP; Requires transport protein; the movement of a substance (solute) from an area of low concentration to an area high concentration against the concentration gradient;
Ch.5 - What type of molecules diffuse easily across the bilayer? (Small-uncharged molecules or large-charged molecules)
easily-Gases and uncharged molecules( Ex. Water (polar)- moderate ; facilitated diffusion by aquaporins)
not-Ions and charged, polar molecules/macro molecules; those require transport proteins and has low permeability
Ch.5 - Phospholipids are said to be amphipathic; what does amphipathic mean?
Amphipathic - both hydrophillic and hydrophobic parts in a molecule [phospholipid has a polar head group(charged region/hydrophillic) and nonpolar tail (uncharged region/hydrophobic)]
Ch.5 - What is osmosis?
the movement of water across membranes to balance solute concentration
Ch.5 - How do plant cells respond in a hypotonic solution? In an isotonic solution? In a hypertonic solution?
hypotonic (turgid/optimal) ; isotonic -flacid; hypertonic - plasmolysis
Ch.5 - What is the optimal solution for a plant cell?
Hypotonic solution - turgid (optimal)
Ch.5 - How do animal cells respond in a hypotonic solution? In an isotonic solution? In a hypertonic solution?
Isotonic solution – optimal (0.9% NaCl)
Hypotonic solution – Osmotic lysis / Hemolysis (too much water/solution and it may burst)
Hypertonic solution - Crenation (shrinks)
Ch.5 - What is the optimal solution for an animal cell?
Isotonic solution (optimal (0.9% NaCl)
Ch.5 - What are the mechanisms of endocytosis and how do they differ?
Phagocytosis - the uptake of solids into the cell ; “eating”
Pinocytosis - the uptake of liquids into the cell; “drinking”
Receptor meditated endocytosis - a ligand binds to a receptor
Ch.6 - How would you draw a graph representation of an exergonic reaction? Label the energy level of the reactants and the products, the Energy of Activation (EA), and the transition state (TS).
Th products have less energy than the reactant
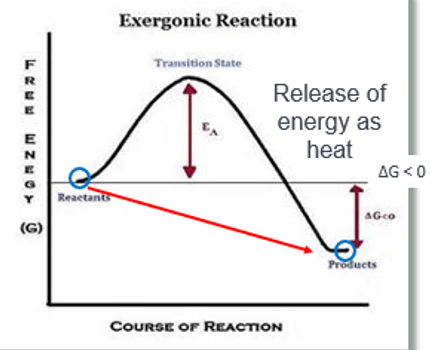
Ch.6 - Is an exergonic reaction spontaneous or non-spontaneous?
spontaneous
Ch.6 - In an exergonic reaction, will the reactants or the products be favored?
reactants
Ch. 6 - In an exergonic reaction, will energy be absorbed or released as heat?
absorbed
Ch.6 - In an exergonic, will the ΔG be positive or negative?
negative
Ch.6 - In an exergonic reaction, how would an enzyme affect this reaction; would it increase the rate of the reaction or decrease it? How?
it will increases the rate of the reaction by reducing the activation energy required for the reaction to proceed
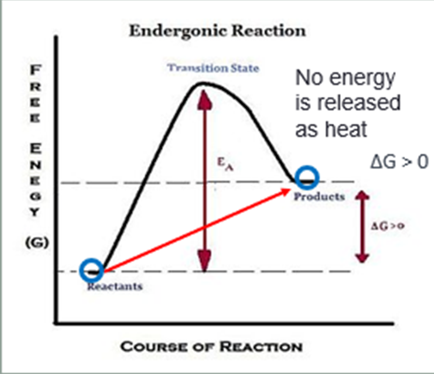
Ch.6 - How would you draw a graph representation of an endergonic reaction? Label the energy level of the reactants and the products, the Energy of Activation (EA), and the transition state (TS).
The products have more energy than the reactants
Ch.6 - Is an endergonic reaction spontaneous or non-spontaneous?
non spontaneous
Ch.6 - In an endergonic reaction, will the reactants or the products be favored?
products
Ch.6 - In an endergonic reaction, will energy be absorbed or released as heat?
released
Ch.6 - In an endergonic, will the ΔG be positive or negative?
positive
Ch.6 - In an endergonic, how would an enzyme affect this reaction; would it increase the rate of the reaction or decrease it? How?
Increase
Ch.6 - A reaction has a ΔG = +3.3 kcal/mol; what can you predict about this reaction?
since ΔG is positive, the reaction is endergonic, and reactants are favored. The reaction will not occur spontaneously because more ender will be required to produce the profucts. all energy will be absorbed in the reaction
Ch.6 - When chemical bonds between atoms are formed, will energy need to be added or released?
released
added
Ch.6 - How are chemical bonds between atoms broken; will energy need to be added or released to break a bond?
Ch.6 - The strength at which a chemical bonds holds two atoms together is referred to as _____________ ________________.
bond strength
Ch.6 - The energy required to break a bond is referred to as __________ _____________.
Bond energy
Ch.6 - What type of energy is stored between the atoms of covalent bonds?
chemical potential energy
Ch.6 - Name two forms of energy that are important to Biology?
light - electromagnetic radiation, packaged in photons
heat - the transfer of kinetic energy from one object to another
Ch.6 - What does it mean when a reaction has met chemical equilibrium?
Ch.6 - What is an oxidation /reduction reaction between atoms?
call redox reaction; it involves the transfer of electrons from one atom to another.
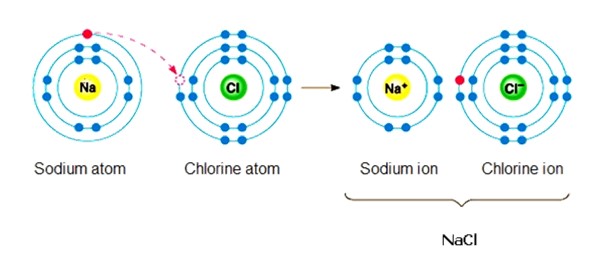
Ch.6 - In the following reaction, has Sodium been oxidized or reduced? Has Chlorine been oxidized or reduced?
sodium has oxidized because it lost an electron
chlorine has reduced because it has gained an electron
Ch.6 - Organic molecules are broken down when they are no longer needed, what is this process called?
catabolic pathway
Ch.6 - Organic molecules are only synthesized from smaller precursor molecule when they are needed, what is this process called?
Anabolic pathway
Ch.6 - What is the difference between a Competitive Enzyme Inhibitor and a Non-Competitive Enzyme Inhibitor?
competitive-they mimic substates and noncovalently bind to the active site of the enzyme and present the “real” substrate from binding
non-competitive - bind to an allosteric site on the enzyme, not the active site; this binding changes the shape of the active site so the substrate cannot bind to the enzyme
Ch.6 - In a catalytic reaction:
Is the enzyme used up in the reaction?
Does the enzyme become part of the reaction?
Does the enzyme become part of the products?
Does the enzyme add energy to the reaction?
Can an enzyme react with many different substrates?
Can an enzyme be denatured? If so, what type of factors will denature them?
Some enzymes work better if they have a “side kick” such as B12 or Zinc; what are these called?
Ch.6 - What are the steps of a catalytic reaction?
Ch.6 - What does the First Law of Thermodynamics state about energy?
Energy cannot be created or destroyed, but it can be transformed from one type to another
Ch.6 - What does the Second Law of Thermodynamics state about the entropy (disorder) of a system as a result of an energy conversion; is entropy increased or decreased?
increased
Ch.6 - What are three types of proteins that use ATP as their source of energy to carry out their function? (Does ATP Synthase use energy?)
Ch.6 - What is a coupled reaction?
an endergonic (non-spontaneous) reaction is coupled with an exergonic reaction (spontaneous) so the endergonic reaction will occur spontaneously
Ch.6 - What type of structure degrades misfolded proteins or proteins that are no longer needed in the cell?
lysosomes
Ch.6 - What type of structure degrades worn out organelles in the cell?
lysosomes
Ch.7 - In what organelle does Cellular Respiration occur?
Mitochondria
Ch.7 - What is the total amount of ATP produced by Cellular Respiration?
38
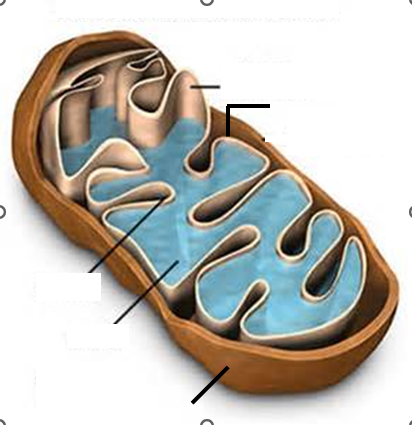
Ch.7 Label the components of the Mitochondria: outer membrane, Inner membrane, cristae, matrix, inter membrane Space.
Ch.7 - What are the steps of Cellular Respiration? Where in the mitochondria does each step occur? What is the net gain of ATP in each step?
1) Glycolysis - occurs in cytosol; Glucose is broken down into 2 Pyruvate; Net gain: +2 ATP per Glucose molecule & +2NADH
2) Breakdown of pyruvate - occurs in matrix (only if O2 is present); Pyruvate to Acetyl-CoA, CO2 is released; Net gain: No ATP, +2 NADH
3) Kreb’s Cycle (aka Citric Acid cycle) - occurs in matrix; Acetyl-CoA to citric Acid, CO2 is released; break down of Glucose is complete; Net gain: 2 ATP, +6 NADH and +2 FADH2
4) Oxidative Phosphorylation and the electron Transport Chain - occurs in the inner membrane; Electron from NADH and FADH are passed through a linked group of protein complexes that together make up the electron transport chain; Net gain: +34 ATP
Ch.7 - In what step if Cellular Respiration is the oxidation of glucose completed?
Kreb cycle
Ch.7 - In what step of cellular respiration of CO2 released as a byproduct?
breakdown of Pyruvate and kreb cycle
Ch.7 - In what step of cellular respiration of Acetyl CoA converted of Citric Acid?
Kreb Cycle
Ch.7 - Is Cellular Respiration an aerobic process or an anaerobic process?
aerobic process
Ch.7 - During Gylcolysis, what is glucose broken down into?
pyruvate
Ch.7 - In what step of Cellular Respiration if CO2 released?
Breakdown of pyruvate and kreb
Ch.7 - Acetyl CoA is concerted to Citric Acid in which step of Cellular Respiration?
kreb
Ch.7 - If oxygen is not present in the cell, what pathway does pyruvate enter? In the process, what is pyruvate converted to that will cause a burning sensation in muscle cells?
no oxygen - does anaerobic respiration and pyruvate enters fermentation pathway and converted into lactic acid/lactate
Ch.7 - What are cristae in the Mitochondria? Why is cristae important in Mitochondria?
highly folded membrane to allow for greater surface area of the membrane. There are proteins embedded in this membrane that are essential to produce ATP
Ch.7 - What is chemiosmosis?
the movement of ions by diffusion across a semipermeable membrane
Ch.7 - What process occurs it oxygen is not present in the cell? Does this process produce any ATP? Name two types of anaerobic repiration.
Anaerobic Respiration
Lactic acid fermentation- Net gain: +2 ATP and Lactate, 2 pyruvate converted to 2 Lactate/lactic acid,
Alcohol fermentation - Glucose is oxidized to 2 pyruvate then broken into CO2 and acetaldehyde, 2 ethanol is produced and secreted from the cell; Net gain: Ethanol and +2ATP
Ch.7 - When a muscle becomes anaerobic during strenuous exercise, pyruvate is converted to __________ _____________.
Lactate
Ch.7 - What is the source of electrons that are passed down the ETC?
NADH & FADH
Ch.7 - During cellular respiration glucose is broken down to produce CO2 and H2O; what is the glucose converted to in each step of oxidation until it cannot be broken any further?
Glucose, Pyruvate, Acetyl CoA, Citric Acid
Ch.8 - Compare and contrast Photosystem I and Photosystem II:
What types of chlorophyll a molecule is found within each PS? What is the length associated ETC of each PS? How is the electron replaced in each PS?
PSI Chlorophyll P700; short ETC
PSII Chlorophyll P680; long ETC; gets electrons from water; replenished by electrons that flow down an electron transport chain from PSII
visible light (380-740nm)
Ch.8 - During photosynthesis, solar energy. the sun is used to produce glucose; what type of solar energy on the electromagnetic spectrum is utilized?
Ch.8 - In what type of cells on the lead are chloroplast located?
Mesophyll cells
Ch.8 - What is the force of energy that drives ATP Synthase?
protons (+H)
Ch.8 - Is oxygen release as a byproduct of the Light Reactions or the Dark reactions of Photosynthesis?
light reactions
light reactions
Ch.8 - Water is a necessary reactant of Photosynthesis. Is water utilized in the Light Reactions or the Dark Reactions?
Ch.8 - When the Calvin Cycle of Photosynthesis is proceeding normally, what enzyme is responsible for carbon fixing CO2 and RuBP to produce 3-PGA?
Rubisco
Ch.8 - During the Reduction phase of the Calvin Cycle, 3-PGA (Phosphoglycerate) is converted to G3P (Glyceraldehyde-3-Phosphate). G3P is then broken down into what organic molecule that the plant needs for growth?
glucose or other carbohydrates
Stroma of chloroplast
Ch.8 - Where in the chloroplast does the Dark Reactions of Photosynthesis occur?
Ch.8 - Where in the chloroplast does the Light Reactions of Photosynthesis occur?
thylakoid membrane
Ch.8 - CO2 is a necessary reactant of Photosynthesis. Is CO2 utilized in the Light Reactions or the Dark Reactions of Photosynthesis?
Dark
Ch.8 - What type of energy is produced via PS I?
NADPH/photon energy
Ch.8 - How is photosynthesis related to cellular? (What does photosynthesis provide that is utilized in cellular respiration? . . .and visa versa?)
Photoautotrophs produce organic molecules glucose and oxygen via photosynthesis; this in turn is used to drive cellular respiration to create ATP and generates CO2 and H2O which drives photosynthesis
Ch.8 - What is an autotroph? Give an example. What is a heterotroph? Give an example.
autotroph - use light as a source of energy (ex. green Plants, algae, cyanobacteria) &
heterotrophs - must consume food to receive energy (ex.bacteria, protists, fungi, animals)
Ch.8 - Do both photosynthesis and cellular respiration occur in the daytime? Do both occur at night?
During daylight, yes and no for night because no sun is out and no sun is needed for cellular respiration.
diffusion
solute move from an area of high concentration to an area of low concentration
oxidation
loss of electron
reduction
light reactions
Split H2O, produces O2, ATP/NADPH, happens in thylakoid membrane, needs light
dark reaction
Uses CO2, produces Glucose, makes ATP/NADPH, rubisco is used to catalyze CO2, happens in the stroma of chloroplast
difference between photosynthesis and cellular Respiration
Photosynthesis converts carbon dioxide and water into oxygen and glucose. Glucose is used as food by the plant and oxygen is a by-product.
Cellular respiration converts oxygen and glucose into water and carbon dioxide. Water and carbon dioxide are by-products and ATP is energy that is transformed from the process.
Kinetic energy
motion enegery
potential energy
stored energy
t or F : In a catalytic enzyme, involving enzymes, does some of the ezymes get used up
F
T
T or F: During the critic cycle, is glucose completely broken down?
T or false: Does there exist an electron flow between cellular respiration and photosynthesis?
T
In the first law of thermodynamics, can energy be destroyed?
No, energy cannot be created nor destroyed
Reactants of cellular respiration
sugar and oxygen
Reactant of photosynthesis
sunlight, water, and carbon dioxide
yes
When an enzyme is denatured, does it lose its shape?
does the mitochondira and the chloroplast have double membranes?
yes
Energy of activation
the amount energy that triggers a reaction
Fermentation
an anaerobic reaction
ph, temperature, deficiency of b12 or b6 (cofactors)
Factors that effect an fucntion enzyme
cellular respiration
an aerobic reaction
Source that drives ATP Synthase to spin
Proton motive force
Glucose
G3P makes….