Lecture 4: Influence of electronic and structural effects on acidity
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
26 Terms
What factors affect acidity?
· Electronegativity
· Inductive effects
· Hybridization
· Resonance / delocalisation
How does electronegativity affect acidity?
more electronegative an element is, the more it helps to stabilise the negative charge of the conjugate base
Trend in acidity for CH₄, NH₃, H₂O, HF?
CH₄ (48) < NH₃ (33) < H₂O (16) < HF (3) — acidity increases with electronegativity.
What is the inductive effect?
Electron‑withdrawing or donating effects transmitted through σ bonds
How do electron‑withdrawing groups affect acidity?
Stabilise conjugate base → increase acidity.
How do electron‑donating groups affect acidity?
Destabilise conjugate base → decrease acidity.
Why is trichloroacetic acid so strong?
Three Cl atoms strongly withdraw electron density → highly stabilised conjugate base.
Which halogen increases acidity the most?
F > Cl > Br > I (due to electronegativity).
Why does inductive effect weaken with distance?
It must be transmitted through σ bonds; effect decays rapidly over multiple bonds.
Strongest electron‑withdrawing groups (in order)?
–NO₂ > –N⁺R₃ > –CN > –CO₂R > –CO– > –OR > –OH.
Most common electron‑donating groups?
Alkyl groups (weak EDGs).
Hybridisation acidity trend?
sp > sp² > sp³ (more s‑character → more acidic).
Why are sp‑hybridised anions more stable?
Lone pair held closer to nucleus → lower energy.
Why is H–C≡N (HCN) more acidic than H–C=CH₂?
Cyanide anion is sp‑hybridised and stabilised by electronegative nitrogen
Why is propiolic acid (pKa 1.9) more acidic than propionic acid (4.9)
sp‑hybridised carbon withdraws electron density more strongly
How does resonance affect acidity?
Delocalisation stabilises the conjugate base → increases acidity.
Why is acetic acid more acidic than ethanol?
Acetate anion is resonance‑stabilised; ethoxide is not.
Carbonyl resonance
The negative charge on the carbanion can delocalize into the carbonyl system
Like charge being spread over pi bond in resonance
In the carbonyl system, the negative charge can be transferred to the oxygen, which is highly electronegative.
Oxygen stabilizes the charge far better than carbon
Resonance in aromatic compounds
A phenyl group exerts an electron‑withdrawing effect because the carbons in the aromatic ring are sp²‑hybridized.
In sp² orbitals, electrons are held closer to the nucleus than in sp³ orbitals, making the ring carbons more electronegative.
As a result, when a phenyl group is attached to a carboxyl group, the bond between them becomes polarized, with electron density pulled toward the aromatic ring.
Why is benzoic acid more acidic than cyclohexanecarboxylic acid?
Aromatic ring (sp² carbons) withdraws electron density inductively.
Why are phenols more acidic than alcohols?
Phenoxide anion is resonance‑stabilised into the aromatic ring.
Why are o‑ and p‑nitrophenols more acidic than m‑nitrophenol?
Nitro group can participate in resonance only at ortho/para positions.
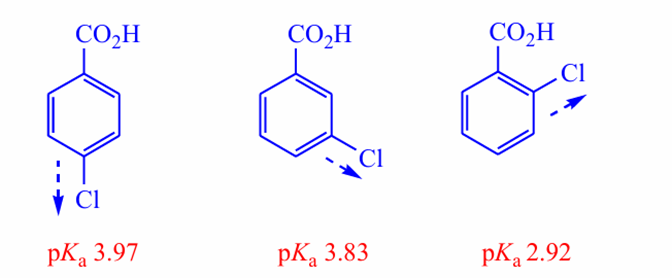
what does o/m/p mean
· Ortho (o-) → the adjacent carbons (directly next to the substituent).
· Meta (m-) → the carbons one carbon away (skip one).
· Para (p-) → the carbon directly opposite the substituent on the ring.
Why is cis‑butenedioic acid more acidic (pKa₁ 1.92) than trans (3.02)?
Intramolecular H‑bonding stabilises the conjugate base.
Why is the second deprotonation of cis‑butenedioic acid harder (pKa₂ 6.23)?
Loss of H‑bond + formation of adjacent negative charges → strong repulsion.
Why is the second deprotonation of trans‑butenedioic acid easier (pKa₂ 4.38)?
Charges are far apart → less repulsion.