R3.4.13 Nitration of benzene
0.0(0)
0.0(0)
Card Sorting
1/9
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
10 Terms
1
New cards
Nitration of benzene: Reactants
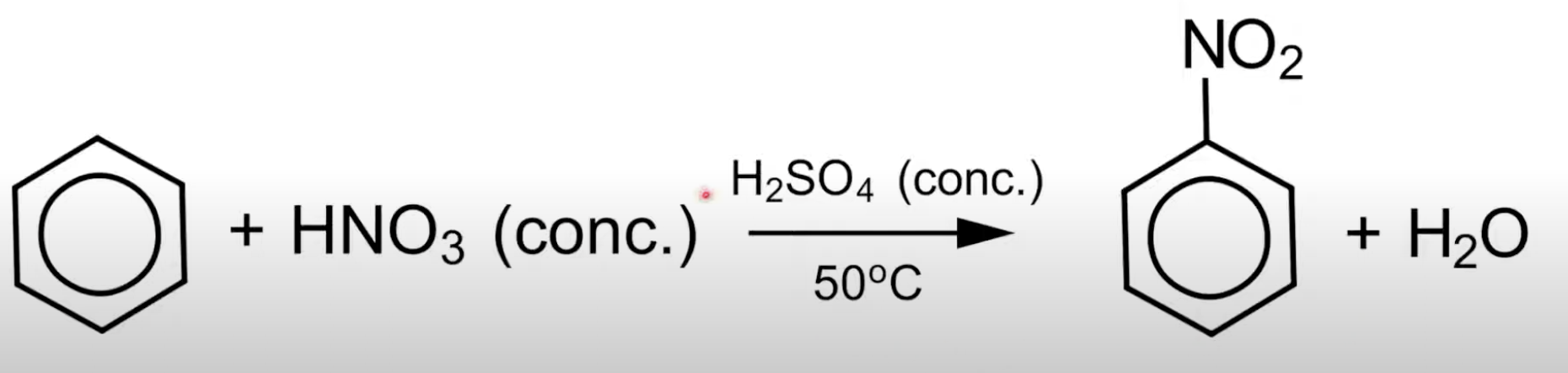
Benzene reacts with concentrated nitric acid and concentrated sulfuric acid to form nitrobenzene and water.
2
New cards
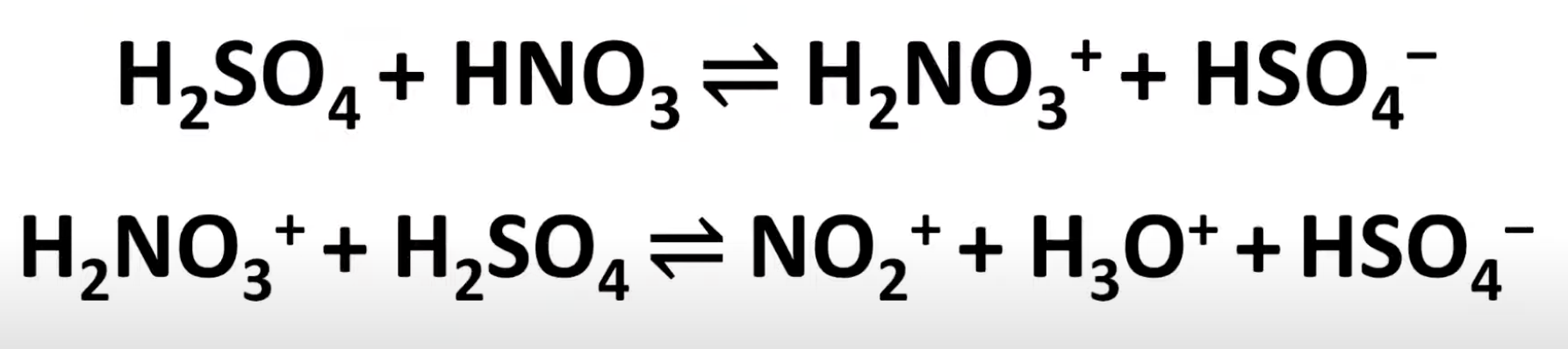
Role of sulfuric acid in nitration
Acts as a catalyst and protonates nitric acid to help generate the nitronium ion.
3
New cards
Electrophile in nitration of benzene
Nitronium ion (NO₂⁺), formed by reaction of nitric acid with sulfuric acid.

4
New cards
Nitronium ion formation
Sulfuric acid protonates nitric acid
Protonated nitric acid loses water to form NO₂⁺.
5
New cards
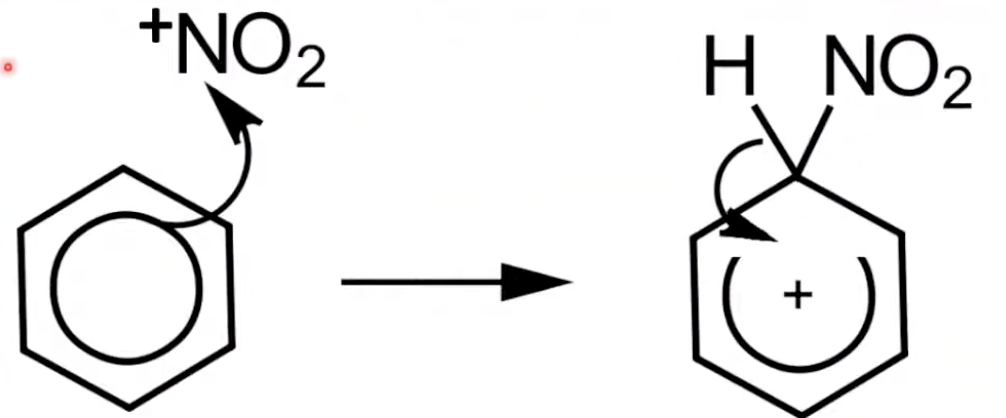
Mechanism step 1: Electrophile attack
NO₂⁺ is attracted to the pi electrons of benzene
Bond forms with a carbon atom, creating a carbocation intermediate.
6
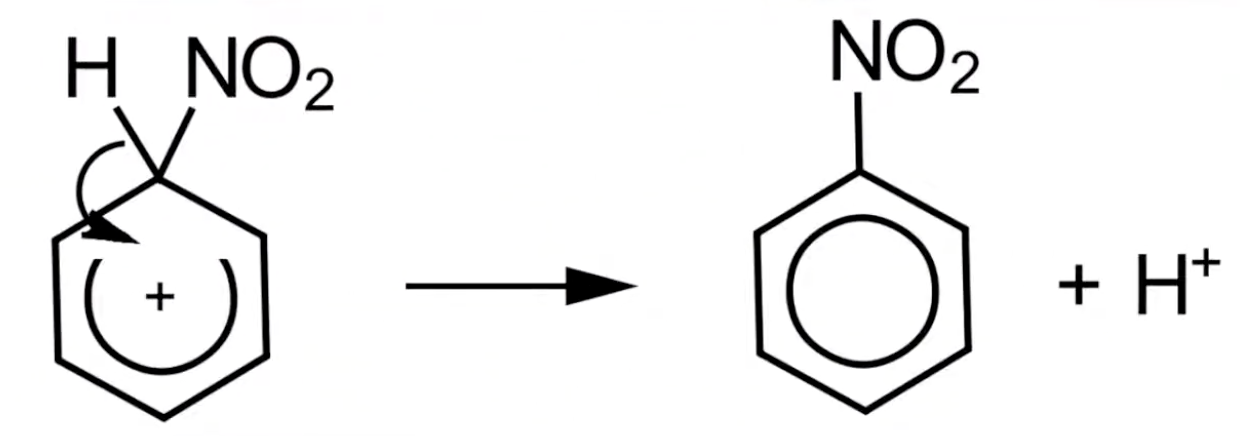
New cards
Mechanism step 2: Reforming benzene ring
A pair of electrons from a C-H bond restore the delocalized pi system, forming nitrobenzene.
7
New cards
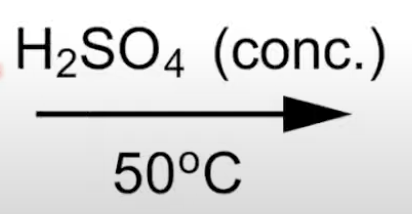
Reaction temperature control
At 50°C, nitrobenzene forms.
Higher temperatures lead to di- or tri-substituted products.

8
New cards
Importance of temperature in nitration
50°C forms nitrobenzene; 65°C forms 1,3-dinitrobenzene
110°C forms 1,3,5-trinitrobenzene.
9
New cards
Overall nitration equation
C₆H₆ + HNO₃ → C₆H₅NO₂ + H₂O (in presence of H₂SO₄ catalyst).
10
New cards
Catalyst regeneration in nitration
H⁺ from the mechanism reacts with HSO₄⁻ to reform H₂SO₄.