Reductions
1/33
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
34 Terms
Reductions with LiAlH4 (LAH)
Reduces esters, acids ketones, aldehydes, etc. to alcohols
Reduces amides, nitriles to primary amines
Rate effects: Highest is LiAlH4 > LiAl(OR)H3, and so on, decreasing reactivity with more alkoxy groups, but higher selectivity
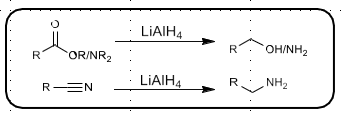
DiBAL-H Reduction
Diisobutyl Aluminum Hydride
Reduces ester to aldehyde or alcohol
Reduces nitrile to aldehyde or amine
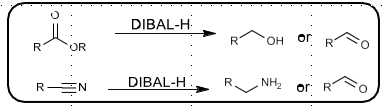
REDAL-H
NaAlH2(OCH2CH2OMe)2
Same reactivity as LiAlH4
Reduces ester/acid, aldehyde, ketone to alcohol
Reduces amide, nitrile to primary amine
Reduces p--toluenesulfonamides to free amines
Alane Reduction
Reduces ester/acid, aldehyde, ketone to alcohol
Reduces amide, nitrile to primary amine
Tolerates halide functional group
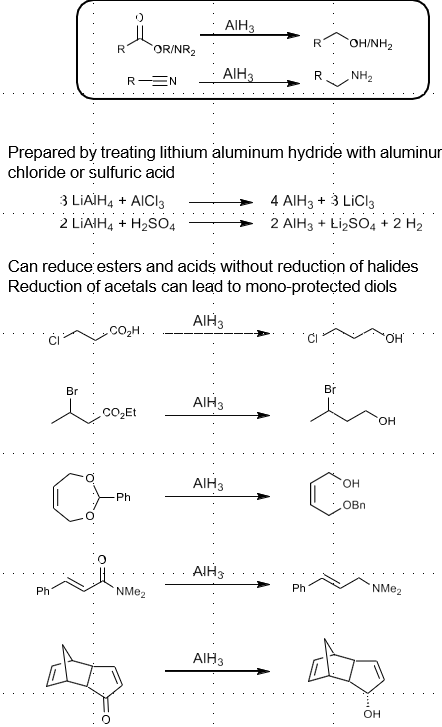
Diborane reduction
Reduces ester/acid, aldehyde, ketone to alcohol
Reduces amide, nitirile to primary amine
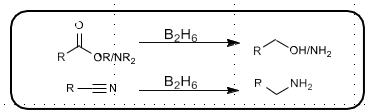
Sodium Borohydride reduction
Mild reducing agent
Reduces aldehydes, ketones to alcohols
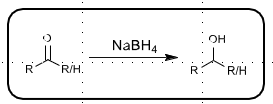
Sodium cyanoborohydride reduction
Used for reductive amination
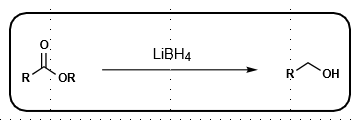
Lithium Borohydride reduction
More reactive than NaBH4, as Li activates oxygen
Reduces esters, aldehydies, ketones to alcohol
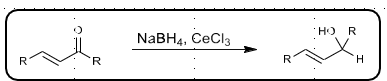
Luche Reduction
Regioselective for 1,2 reduction of alpha-beta unsaturated ketones
CeCl3 acts as LA
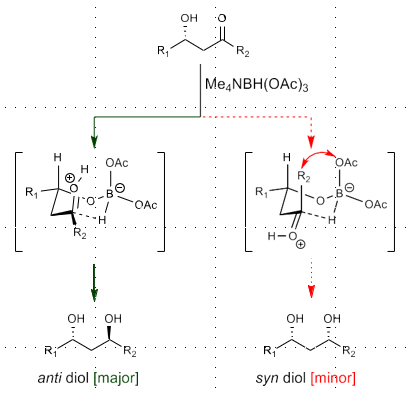
Sodium Trialkoxyborohydride(Internal H- delivery)
NaBH(OAc)3/Me4NBH(OAc)3
Requires chiral OH directing group
Gives rise to 1,3 anti-selectivity
Via Chair transition state, anti product minimizes A 1,3 interactions
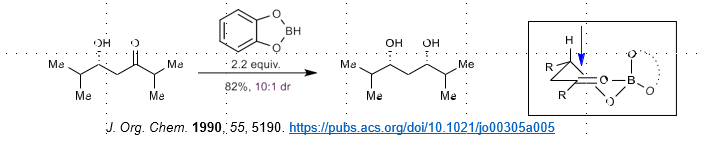
Narasaka-Evans Reduction (external H- delivery)
Requires chiral OH directing group
Uses catechol boranes + external hydride source
Gives rise to 1,3 syn-selectivity
L/K-selectride reduction
Reduces ester/acid, aldehyde, ketone to alcohol
Reduces amide, nitrile to primary amine
Large, bulky hydride sources
Preferential for 1,4 reduction. Resulting enolate can be trapped with electrophiles
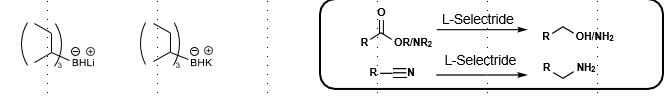
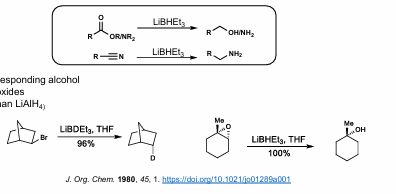
LiBHEt3 (Super Hydride Reduction)
Reduces Ester/acid to alcohol
Reduces amide, nitrile to primary amine
Reduces HALOGENS and can OPEN EPOXIDES
VERY powerful reducing agent (stronger than LiAlH4)
Burgi-Dunitz Angles
Angles for nucleophilic attack at carbonyl
sp2 (carbonyl): 105 degrees
sp3 (SN2): 180 degrees
sp: 120 degrees
Baldwin’s Rules
Rules for ring formation
Broken bound outside cycle: “exo”
Broken bond inside cycle": “endo”
Based on ring size:
Favored:
Tet (sp3):
3 to 7: exo-tet favored
Trig (sp2):
3 to 7 exo-trig are favored
6 to 7 endo-trig are favored
Dig (sp)
3 to 7 endo-dig are favored
5 to 7 endo-dig are favored
Disfavored:
Tet (sp3):
5 to 6: endo-tet disfavored
Trig (sp2):
3 to 5 endo-trig are disfavored
Dig (sp):
3 to 4 exo-dig are disfavored
Felkin-Ahn Model
Put carbonyl 90 degrees in Newman projection, attack between Rm and Rs
Electronegative atom:
When there is an alpha electronegative atom/group, when electronegative atom is perpendicular to carbonyl, most reactive confomer. EN ATOMS REPLACE RL
Larger R groups (substituent on carbonyl) increases preference for Felkin pdt
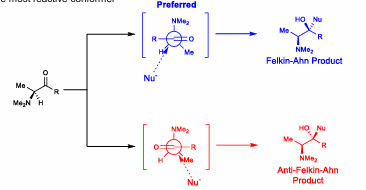
Cram model
Same and Felkin-ahn, but chiral directing groups lock conformer via chelation. Attack occurs via least hindered site.
Good chelators: Mg, Zn, Li, Ti, Sn, Al
Bad chelators: Na, K, BF3
O-sub can invert stereochemistry (large groups can give Felkin-Ahn pdt)
1,2 Cram Chelation: Anti
1,3 Carm Chelation: Syn
Reduction of cyclohexanones
Small hydride: Axial Attack. Why? Torsional strain overcomes axial sterics
Large hydride: Equatorial attack. Why? Axial sterics overpower torsional strain
Reduction of bicyclic systems
Nu will choose convex face (open face) due to less steric interactions
Enantioselective Reductions
Chiral moeities in molecule, BINOL + LiAlH —> BINAL
Alpine-Borane Reduction
Enantioselective Reduction of Borane
Derived from Hydroboration of alpha-pinene
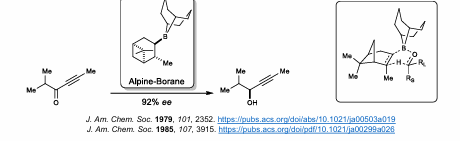
DIP-Chloride
B-chlorodiisopinocampheylborane
Highly enantioselective
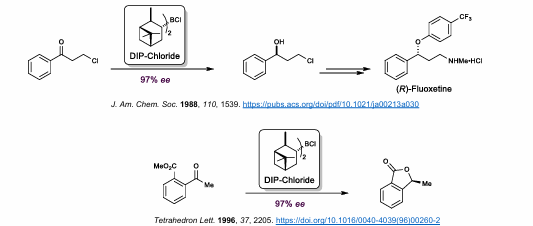
Corey-Itsuno/Corey-Bakshi-Shibata (CBS) Reduction
Enantioselective reduction of ketones, aldehydes
Chiral catalyst prepared from proline
Meerwein-Pondorf-Verley (MPV) Reduction
Reversible Reduction using Al(OiPr)3
Thermodynamic product is obtained
Axial delivery preferred

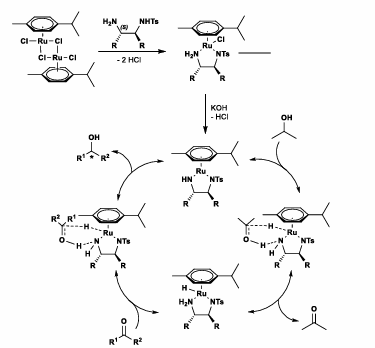
Noyori Transfer Hydrogenation
Ru chiral catalyst
Isopropanol as Hydride source
Enantioselective reduction of Ketones, aldehydes
Can also have Formic acid/triethylamine as hydride source
In a Beta-keto ester, ketone is reduced preferentially
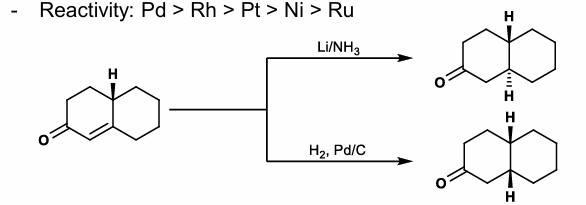
Least Hindered Catalytic Hydrogenation
Cis-delivery from least hindered face of olefin
Reactivity:
Pd > Rh > Pt > Ni > Ru
alkyne > terminal olefin > single sub > double > triple
Lindlar’s catalyst gives cis olefins (Pd(BaSO4), H2) from alkynes
Birch conditions gives trans
Common catalysts: Pd/C, Pd(OH)2 (Pearlmann’s cat), PtO2 (Adams’ cat) for imines to amines, Rainey Ni for sulfide groups,
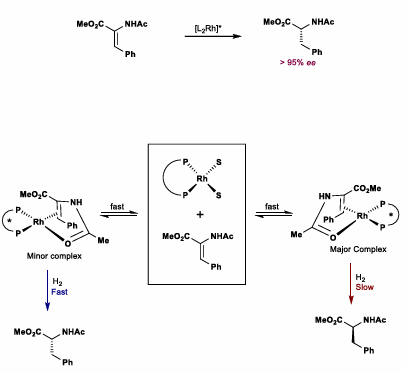
Wilkinson’s Catalyst
Most hindered face hydrogenation (minor complex reacts fast)
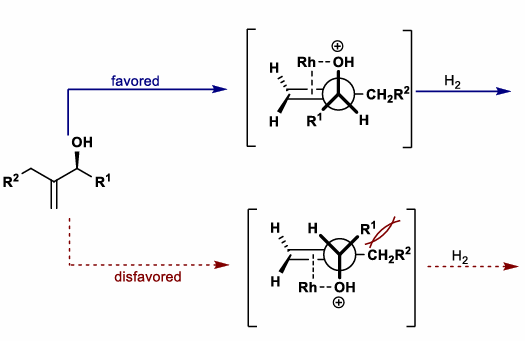
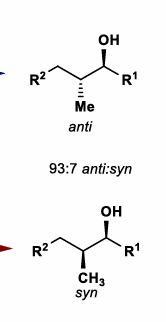
Hydrogenation of Allylic and Homoallylic Alcohols
Draw conformation of allylic alcohol with alcohol perpendicular to alkene (Felkin-Ahn like)
Determine favored conformation based on sterics
Favored conformation determines reduction stereoselectivity
For CH2OH groups (i.e non-OH group), perform same process
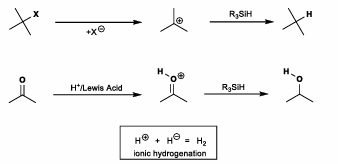
Ionic Reduction
Requires good LG, forms carbenium. Hydride transfer affords hydrogenated product.
Can also have Lewis acid plus hydride transfer.
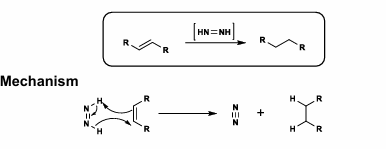
Diimide Reduction
[HN=NH reagent
Cis-delivery of H2 on least hindered face
Trans>Cis olefin rate
Carbonyls, nitro, nitrile, sulfoxide, disulfide all tolerated
ONLY olefin reduction? Diimide is probably the way to go
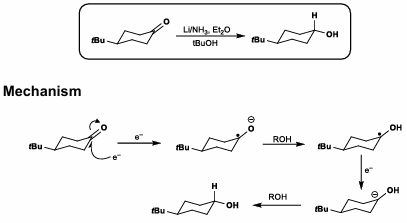
SET Reductions
Birch conditions (Li/NH3, ether solvent)
Most thermodynamically stable product is given
Trans-fused decalins are made, as hydrogenation typically gives cis-fused
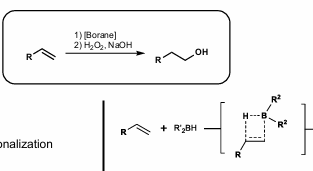
Hydroboration
Anti-Markovnikov selectivity
Can oxidize to Anti-Markovnikov alcohol
4-membered sigma-metathesis transition state
Larger borane = better regioselectivity
Increased rate:
Higher substitution on olefin
More olefin strain
Decreased rate:
Steric bulk on olefin
Regioselectivity based on electronic factors:
EDG stabilizes positive charge, gives better selectivity
EWG destabilizes positive charge, gives worse selectivity
TMS stabilizes positive charge (beta-silicon effect), but can give worse selectivity.
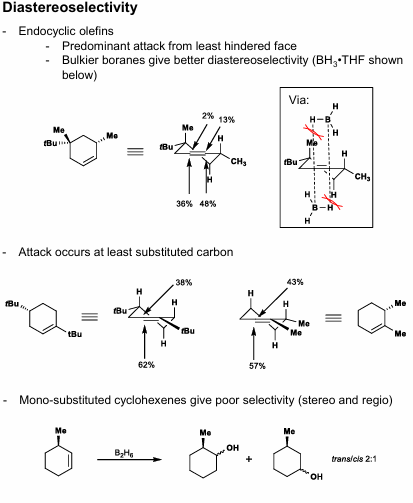
Hydroboration Diastereoselectivity (Endocylic olefins)
Diastereoselectivity
(Endocyclic olefins):
Least-hindered face is attacked
Bulkier boranes give better diastereoselectivity
Attack occurs at least substituted carbon
Basically, least substituted carbon that forms a chair transition state
Inductive effect: Methoxy group destabilizes positive charge up at adjacent carbon, 1,2 product is favored (as opposed to 1,3)
Larger boranes give better stereoselectivity but reduced regioselectivity
(Exocylic):
Small borane = axial
Large borane = equatorial
Hydroboration Diastereoselectivity (Acyclic)
(Acyclic olefins):
Draw reactive conformations of olefin
RL (large sub) perpendicular to olefin, opposite to side of Boron attack
Borane approaches from opposite face
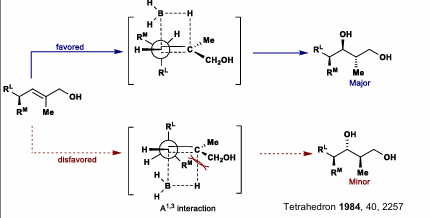
For 1,1 disubstituted olefins: Diastereoselectivity is driven by A1,2 strain
For dialkylboranes, the diastereoselectivity is driven by interactions between R groups on boron and Rm (medium size sub on alpha carbon). Dialkyl interactions is less favored than A1,2 for dialkylboranes.
Remember, place RL 90 degrees to double bond (2 configurations, one with RL up and one down). Let borane attack from opposite face. Whatever you get, that is the major product.