chemistry - separate chemistry 2: hydrocarbons (9.10 - 9.16)
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
24 Terms
9.10 methane - formula
CH4
9.10 methane - structure
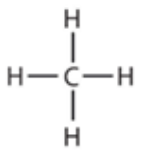
9.10 ethane - formula
C2H6
9.10 ethane - structure
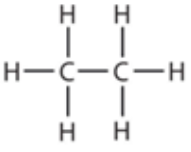
9.10 propane - formula
C3H8
9.10 propane - structure
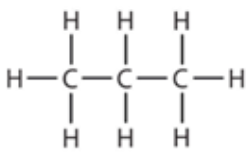
9.10 butane - formula
C4H10
9.10 butane - structure
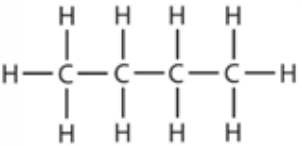
alkane general formula
CnH2n+2
alkene general formula
HnC2n
9.11 why are alkanes saturated hydrocarbons?
carbon atoms joined by single covalent bonds (C-C)
9.12 ethene - formula
C2H4
9.12 ethene - structure
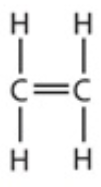
9.12 propene - formula
C3H6
9.12 propene - structure
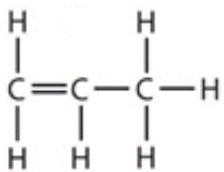
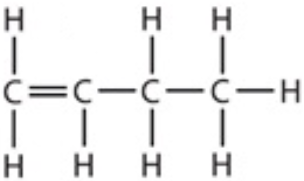
9.12 butene - formula
C4H8
9.12 but-1-ene - structure
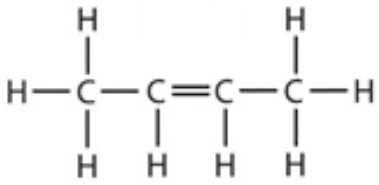
9.12 but-2-ene - structure
9.13 why are alkenes unsaturated hydrocarbons?
contain functional group C=C (carbon-carbon double bond)
9.14 ethene + bromine →
1,2-dibromoethane
9.14 ethene + bromine - structures of reactants & products
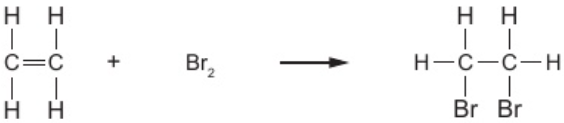
9.14 ethene + bromine - other alkenes
1,2-dibromoethane:
“di”: means “two”
“1,2”: 2 bromine atoms attached to diff. carbon atoms
if attached to same carbon atom - “1,1-dibromoethane”
9.15 bromine water - distinguish between alkanes & alkenes
bromine water = orange-brown
bromine + alkene: C=C double bond reacts with bromine → bromine removed from solution → forms colourless product
bromine + alkane: no decolourisation - remains brown
9.16 complete combustion of alkanes & alkenes
CO2 + water produced
hydrocarbons oxidised