Midterm Two: Ch.7 Chemical Reactions and Equations
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
23 Terms
Strong Bases
metal hydroxide
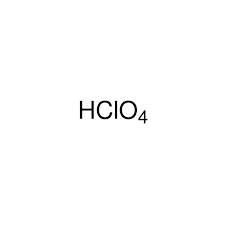
Strong Acid: HClO4(aq)
Perchloric Acid
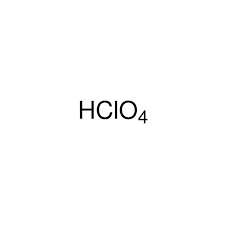
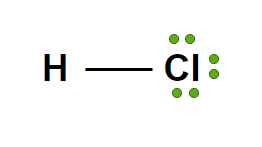
Strong Acid: HCl(aq)
Hydrochloric acid
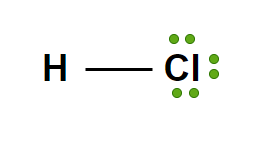
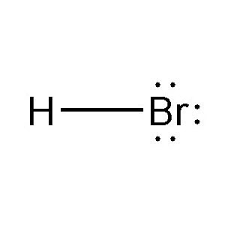
Strong Acid: HBr(aq)
Hydrobromic Acid
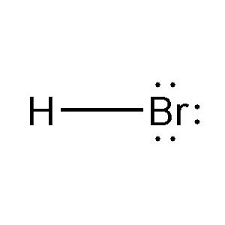

Strong Acid: HI(aq)
Hydroiodic Acid


Strong Acid: H2SO4(aq)
Sulfuric Acid

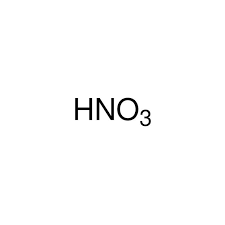
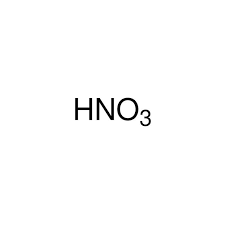
Strong Acid: HNO3(aq)
Nitric Acid
Decomposition Rx of carbonic acid: H2CO3(aq)
carbon dioxide + water
CO2(g) + H2O(l)
Decomposition Rx of sulfurous acid: H2SO3(aq)
sulfur dioxide + water
SO2(g) + H2O(l)
Decomposition Rx of ammonium hydroxide: NH4OH
ammonia + water
NH3(g) + H2O(l)
Combination Rex
A+B —> AB
Decomposition Rx
AB —> A+B
Single Replacement Rx
A + BC —> B + AC
If A is more reactive than B
Double Replacement (Displacement) Rx
AB + CD —> AD + BC
DR Rx: Precipitation
Solid formation reactions, if all reactants and products are aqueous, write NR
DR Rx: Acid Base (neutralization, heat formation)
Acid + base —> salt + water + heat
DR Rx: strong acid to weak acid/ strong base to weak base (gas formation)
Acids and bases memorized
Combustion Rx
Hydrocarbon + oxygen gas —> carbon dioxide gas + water + heat
Redox (Reduction oxidation) Rx
Oxidation = loss of e
Reduction = gain of e
limiting reactant
the thing that is not in excess; cannot be the product
formula for percent yield
(experimental yield/ theoretical yield) x 100
empirical rule
represents the lowest whole number ratio of atoms in a compound
Molar mass
Mm= g/mol