Shapes of molecules and ions
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
10 Terms
What is the electron-pair repulsion theory
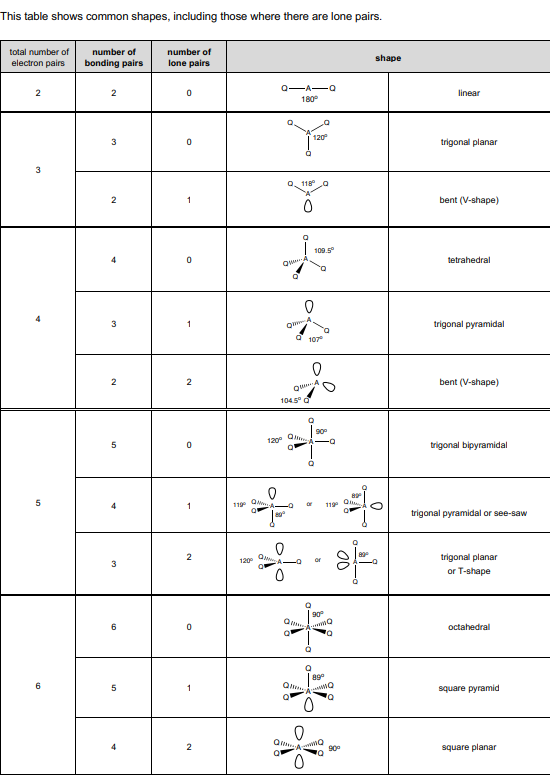
As electrons are negatively charged they repel each other so they are arranged as far away from each other. Arrangement of electron pairs minimises repulsion, different number of electron pairs results in different shapes.
Note:
Electron pairs surrounding central atom determine the molecules shape.
Describe lone-pair and bonded-pair repulsion
Lone pairs are closer to the central atom than bonded pairs so repel more strongly than them. This pushes bonded pairs closer together closer together, which decreases the bond angle.
Note:
A double bond is treated the same as a single bond
How much do lone pairs take off the bond angle
Take off 2.5o
What does each wedge represent

If there is only two bonding pairs what is the molecule shape and bond angle
Linear and 180o
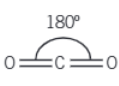
If there is only three bonding pairs what is the molecule shape and bonding angle
Trigonal and 120o
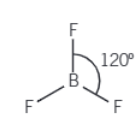
If there is 3 electron pairs, two which are bonding pairs and one lone pair what is the molecule shape and bonding angle
Bent (V-shape) and 118o
This molecule has only 4 bonding pairs, shape and bond angle?
Tetrahedral 109.5o
This molecule has 4 electron pairs one of which is a lone pair others are bonded shape and bond angle?
Trigonal pyramidal 107o
Table showing shapes of molecules and bond angles