Core Practical 5: The oxidation of ethanol
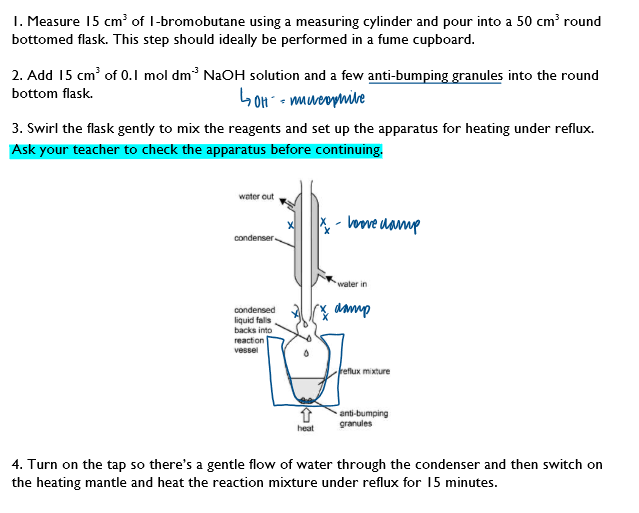
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
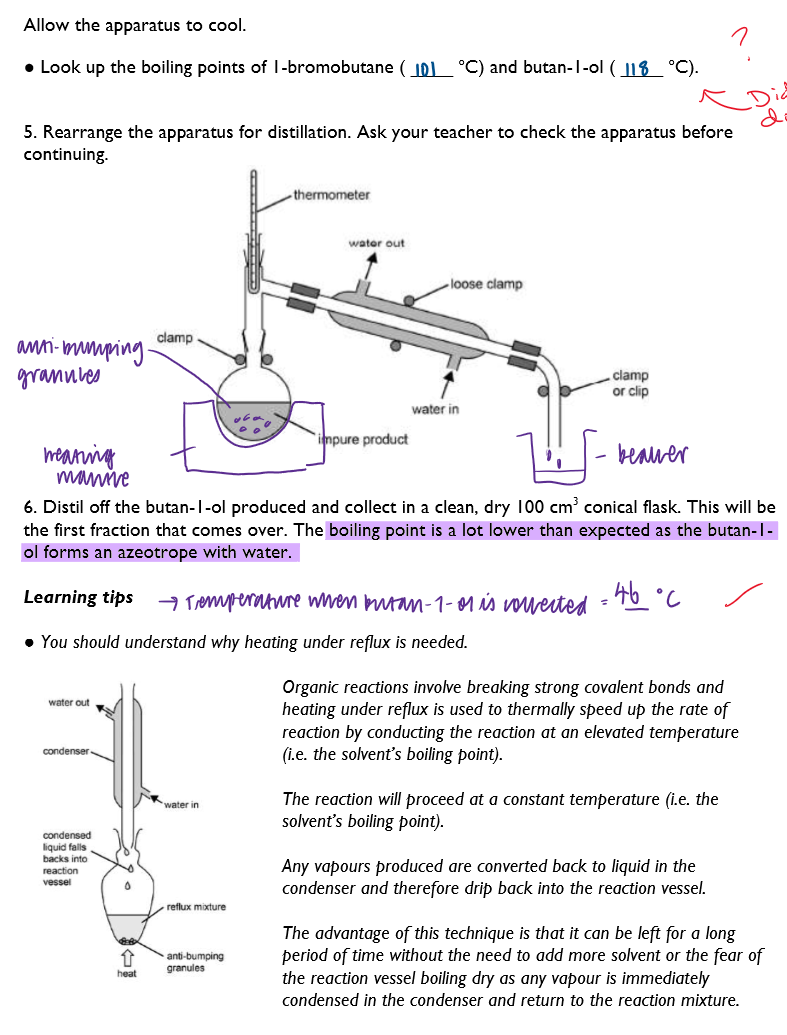
|---|
No analytics yet
Send a link to your students to track their progress
9 Terms
Overview:

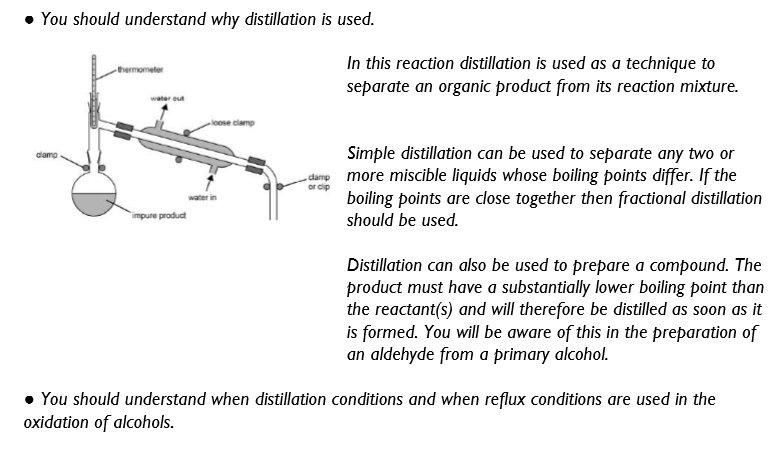
Procedure:
Procedure:
N.B.
Write an ionic equation for the conversion of 1-bromobutane into butan-1-ol.
N.B. use structural formulae
CH3CH2CH2CH2Br (l) + OH- (aq) → CH3CH2CH2CH2OH (l) + Br-(aq)
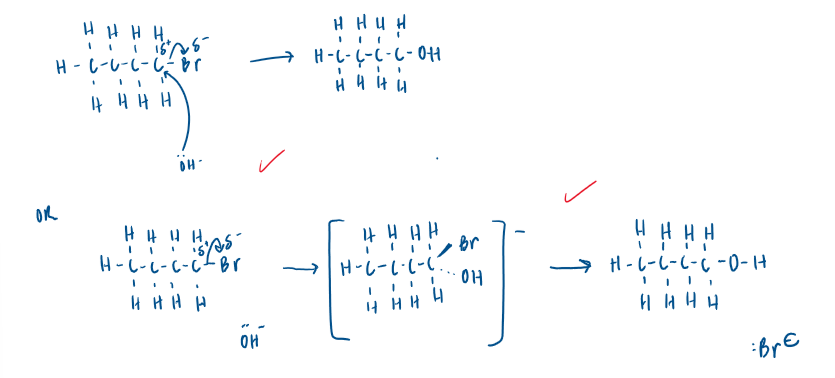
Draw a mechanism for the reaction and suggest why you have chosen this mechanism.
1-bromobutane is a primary haloalkane therefore would react via the SN2 mechanism forming temporary bonds
Describe the tests that will confirm (or otherwise) the presence of:
(i) butan-1-ol in the distillate
acidified potassium dichromate, changes from orange to green if alcohol is present
Describe the tests that will confirm (or otherwise) the presence of:
(ii) unreacted bromoalkane
first add nitric acid to neutralise NaOH
add aqueous AgNO3
a cream ppt would form if bromoalkane was present
Ag+ + Br- → AgBr
Analysis of the product shows that there is still unreacted 1-bromobutane present. What modifications could you make to the method to try and improve the conversion to butan-1-ol?
heat the reaction mixture under reflux for a longer period
use a higher concentration of NaOH at higher temperatures
use an excess of NaOH