Orgo CH12 Practice Problems
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
13 Terms
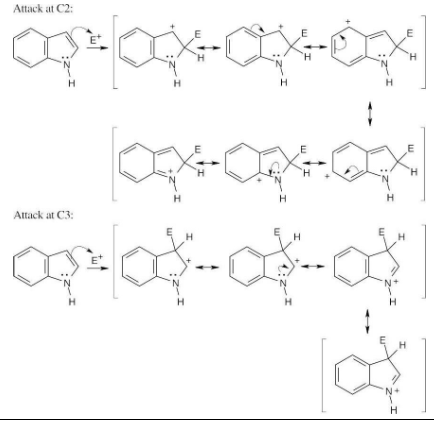
Name the following compounds:
Facts to remember about naming amines:
(1) Primary amines are named by adding the suffix -amine to the name of the alkyl substituent.
(2) The prefix di– or tri– is added to the names of symmetrical secondary and tertiary amines.
(3) Unsymmetrical secondary and tertiary amines are named as N-substituted primary amines. The parent amine has the largest alkyl group.
(4) Heterocyclic amines have unique parent names; the heteroatoms have the lowest possible numbers.
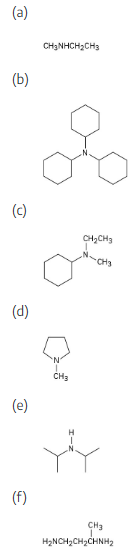
(a) CH3NHCH2CH3
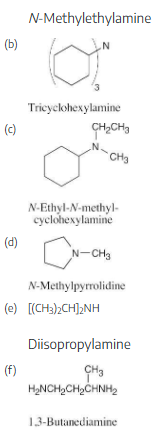
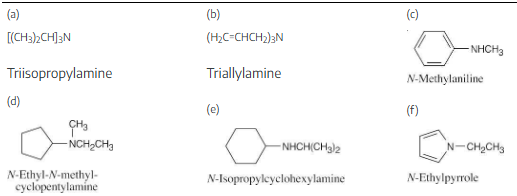
Draw structures corresponding to the following IUPAC names:
(a) Triisopropylamine
(b) Triallylamine
(c) N-Methylaniline
(d) N-Ethyl-N-methylcyclopentylamine
(e) N-Isopropylcyclohexylamine
(f) N-Ethylpyrrole
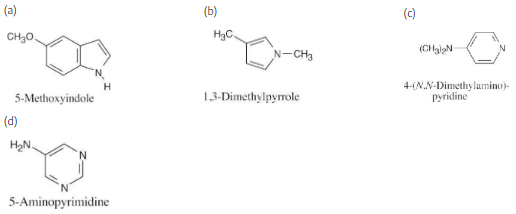
Draw structures for the following heterocyclic amines:
(a) 5-Methoxyindole
(b) 1,3-Dimethylpyrrole
(c) 4-(N,N-Dimethylamino)pyridine
(d) 5-aminopyrimidine
The numbering of heterocyclic rings is described in Section 12.1.
Which compound in each of the following pairs is more basic?
(a) CH3CH2NH2 or CH3CH2CONH2
(b) NaOH or CH3NH2
(c) CH3NHCH3 or pyridine
Amines are less basic than hydroxide but more basic than amides. The pKa values of the conjugate acids of the amines in (c) are shown. The larger the pKa, the stronger the base.

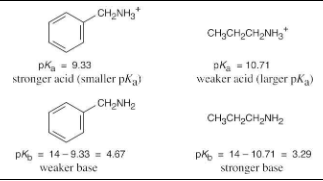
The benzylammonium ion (C6H5CH2NH3+) has pKa = 9.33, and the propylammonium ion has pKa = 10.71. Which is the stronger base, benzylamine or propylamine? What are the pKb’s of benzylamine and propylamine?
The stronger base (propylamine) holds a proton more tightly than the weaker base (benzylamine). Thus, the propylammonium ion is less acidic (larger pKa) than the benzylammonium ion (smaller pKa).
To calculate pKb: Ka · Kb = 1 x 10–14, pKa + pKb = 14 and pKb = 14 – pKa.
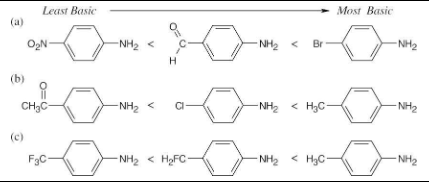
Without looking at Table 12.4, rank the following compounds in order of ascending basicity.
(a) p-Nitroaniline, p-aminobenzaldehyde, p-bromoaniline
(b) p-Chloroaniline, p-aminoacetophenone, p-methylaniline
(c) p-(Trifluoromethyl)aniline, p-methylaniline, p-(fluoromethyl)aniline
The basicity order of substituted arylamines is the same as their reactivity order in electrophilic aromatic substitution reactions because, in both cases, electron-withdrawing substituents make the site of reaction more electron-poor and destabilize a positive charge.
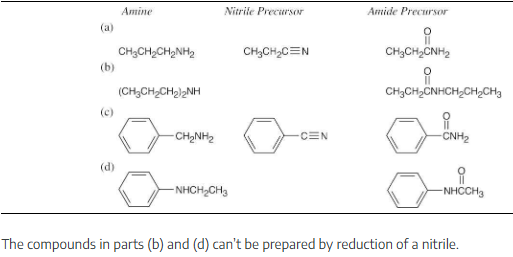
Propose structures for either a nitrile or an amide that might be a precursor of each of the following amines:
(a) CH3CH2CH2NH2
(b) (CH3CH2CH2)2NH
(c) Benzylamine, C6H5CH2NH2
(d) N-Ethylaniline
Amide reduction can be used to synthesize most amines, but nitrile reduction can be used to synthesize only primary amines. Thus, the compounds in (b) and (d) can be synthesized only by amide reduction.
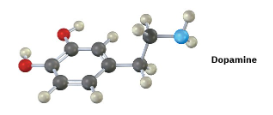
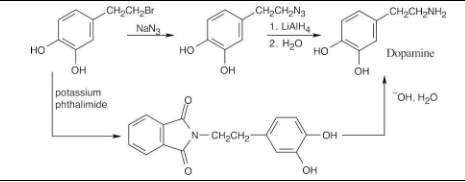
Show two methods for the synthesis of dopamine, a neurotransmitter involved in regulation of the central nervous system. Use any alkyl halide needed.
The upper reaction is the azide synthesis, and the lower reaction is the Gabriel synthesis.
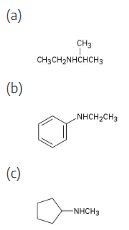
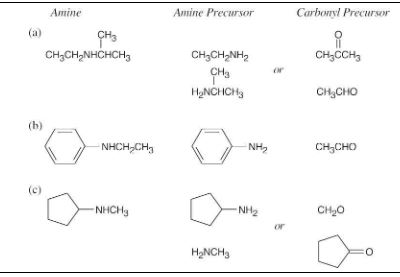
How might the following amines be prepared using reductive amination reactions? Show all precursors if more than one is possible.
Look at the target molecule to find the groups bonded to nitrogen. One group comes from the aldehyde/ketone precursor, and the other group comes from the amine precursor. In most cases, two combinations of amine and aldehyde/ketone are possible.
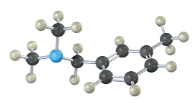
How could you prepare the following amine using a reductive amination reaction?
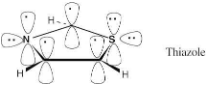
Draw an orbital picture of thiazole. Assume that both the nitrogen and sulfur atoms are sp2-hybridized, and show the orbitals that the lone pairs occupy.
Thiazole contains six π electrons. Each carbon contributes one electron, nitrogen contributes one electron, and sulfur contributes two electrons to the ring π system. Both sulfur and nitrogen have lone electron pairs in sp2 orbitals that lie in the plane of the ring.
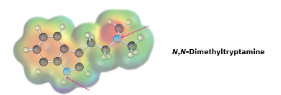
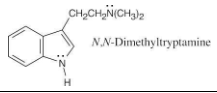
Which nitrogen atom in the hallucinogenic indole alkaloid N,N-dimethyltryptamine is more basic? Explain.
The side chain nitrogen atom of N,N-dimethyltryptamine is more basic than the ring nitrogen atom because its lone electron pair is more available for donation to a Lewis acid. The aromatic nitrogen electron lone pair is part of the ring π electron system.
Indole reacts with electrophiles at C3 rather than at C2. Draw resonance forms of the intermediate cations resulting from reaction at C2 and C3, and explain the observed results.
Positive charge can be stabilized by the nitrogen lone-pair electrons in reaction at both C2 and C3. In reaction at C2, however, stabilization by nitrogen destroys the aromaticity of the fused benzene ring. Reaction at C3 is therefore favored, even though the cationic intermediate has fewer resonance forms, because the aromaticity of the six-membered-ring is preserved.