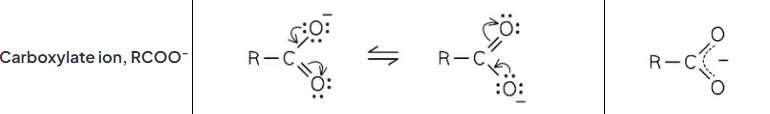2.2.11 Resonance Structures
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
10 Terms
what are delocalised electrons
electrons in a molecule, ion or metal that are not associated with one atom or one covalent bond
when do resonance structures exist
when there is more than one possible lewis structure that can be drawn for the position of a multiple bond
what is the bond length and strength in a resonance structure
intermediate in length and strength between a double and triple bond
what is the actual structure of the combination of all resonance structures known as
resonance hybrid
what must a molecule have to form a resonance hybrid strucutre
a double bond ( a pi bond) capable of migrating from one part to another
when 2 occasions does this usually arise
when adjacent atoms with equal electronegativity (same atoms)
lone pair of electrons can re arrange themselves
draw the lewis resonance structures of carbonate ion and resonance hybrid
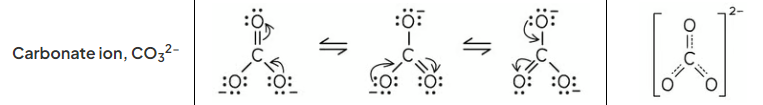
how does the resonance structure of carbonate ion arise
the c=o bond is in a different position becuase the lone pair of electrons on the oxygen atom overlap with the pi electrons from the c=o bond and so 4 pi electrons are shared
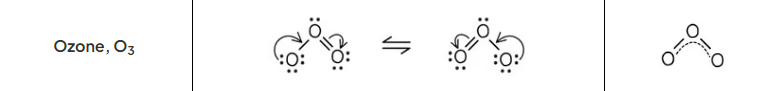
draw the lewis resonance structures and resonance hybrid of ozone
draw the resonance structure of the carboxylate ion