Effect of temperature on reaction rate using sodium thiosulfate and hydrochloric acid
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
11 Terms
Step 1
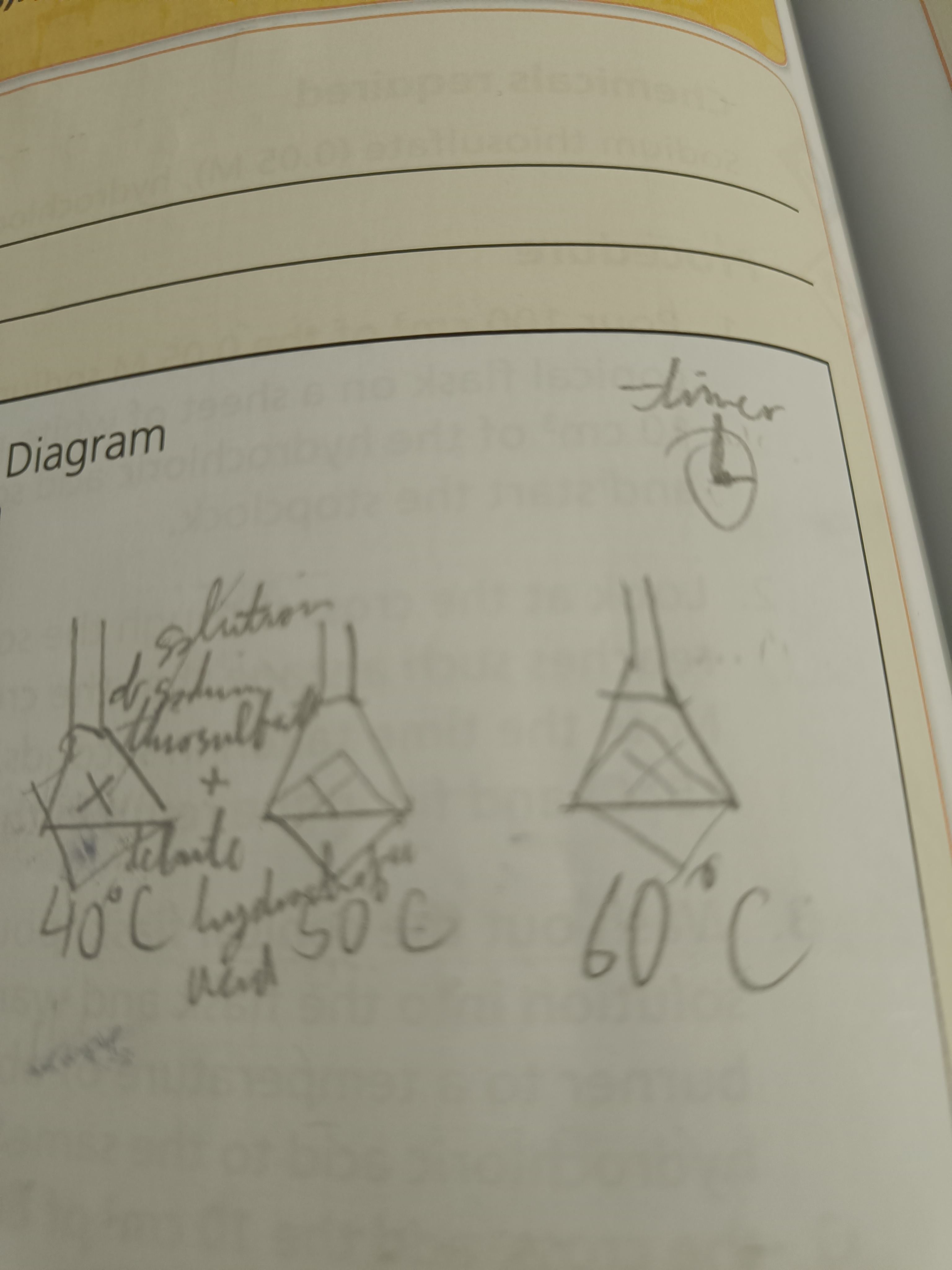
Set up apparatus as shown and start the clock once the hydrochloric acid and the flask is swirled
Step 2
When the degree of milkiness reaches a point where the cross isn’t visible stop the timer and measure the temperature.
Step 3
Repeat these steps but heat the sodium thiosulfate and hydrochloric acid to various temperatures
Draw a diagram of this experiment
…
Why is a dilute solution of sodium thiosulfate used in this experiment?
So that the measured times will not be too short at higher temperatures
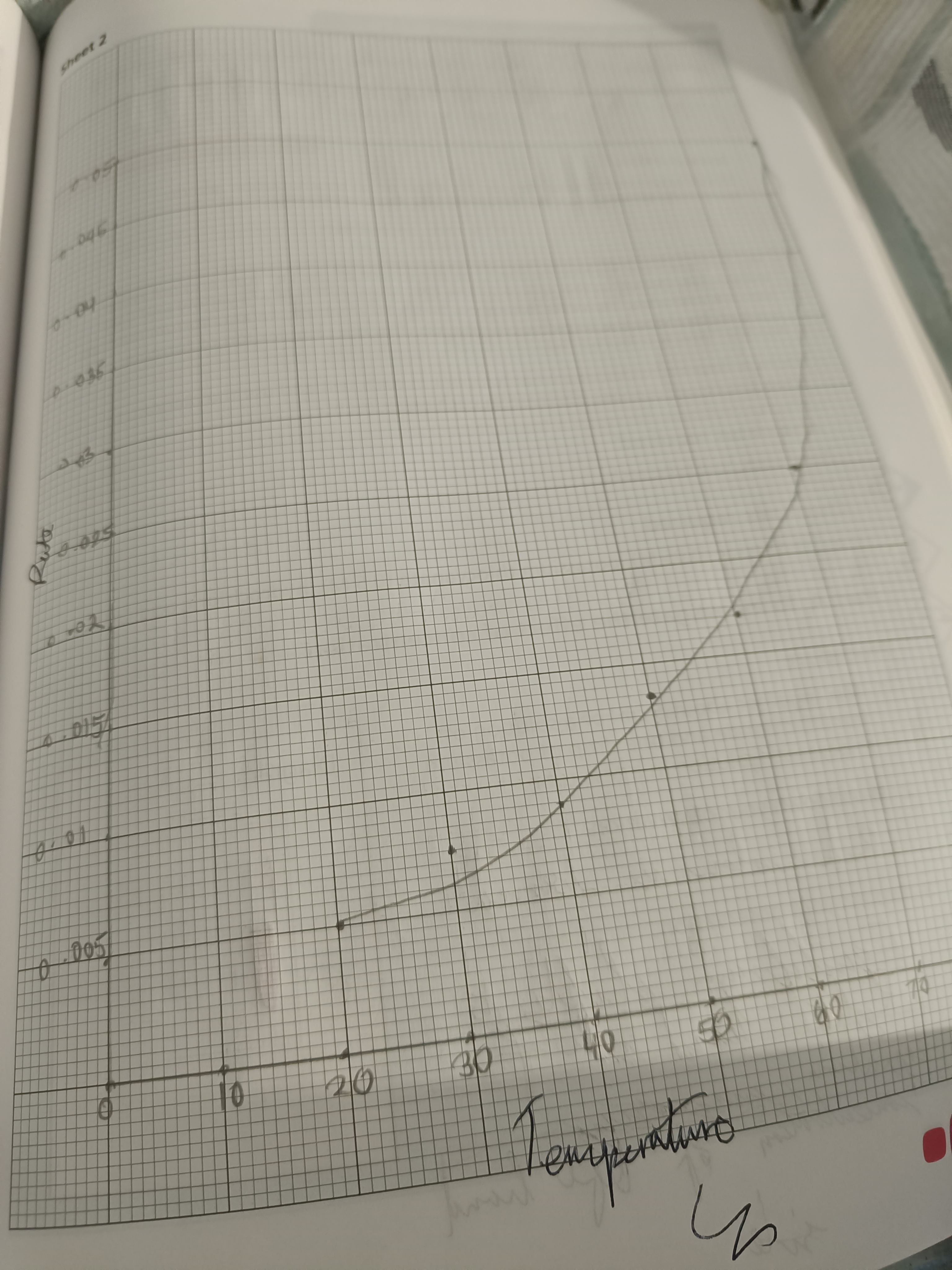
Graph for concentration vs rate is linear, why is the temp vs rate graph a curve?
Rate of reaction is not directly proportional to the temperature
Two everyday examples of temperature affecting the rate of a reaction
Food in fridge’s cool temp slows down decomposition. Catalytic converter works best when heated.
What gas is released when sodium thiosulfate reacts with HCl?
SO2
Describe the smell of SO2
Irritating/strong smell
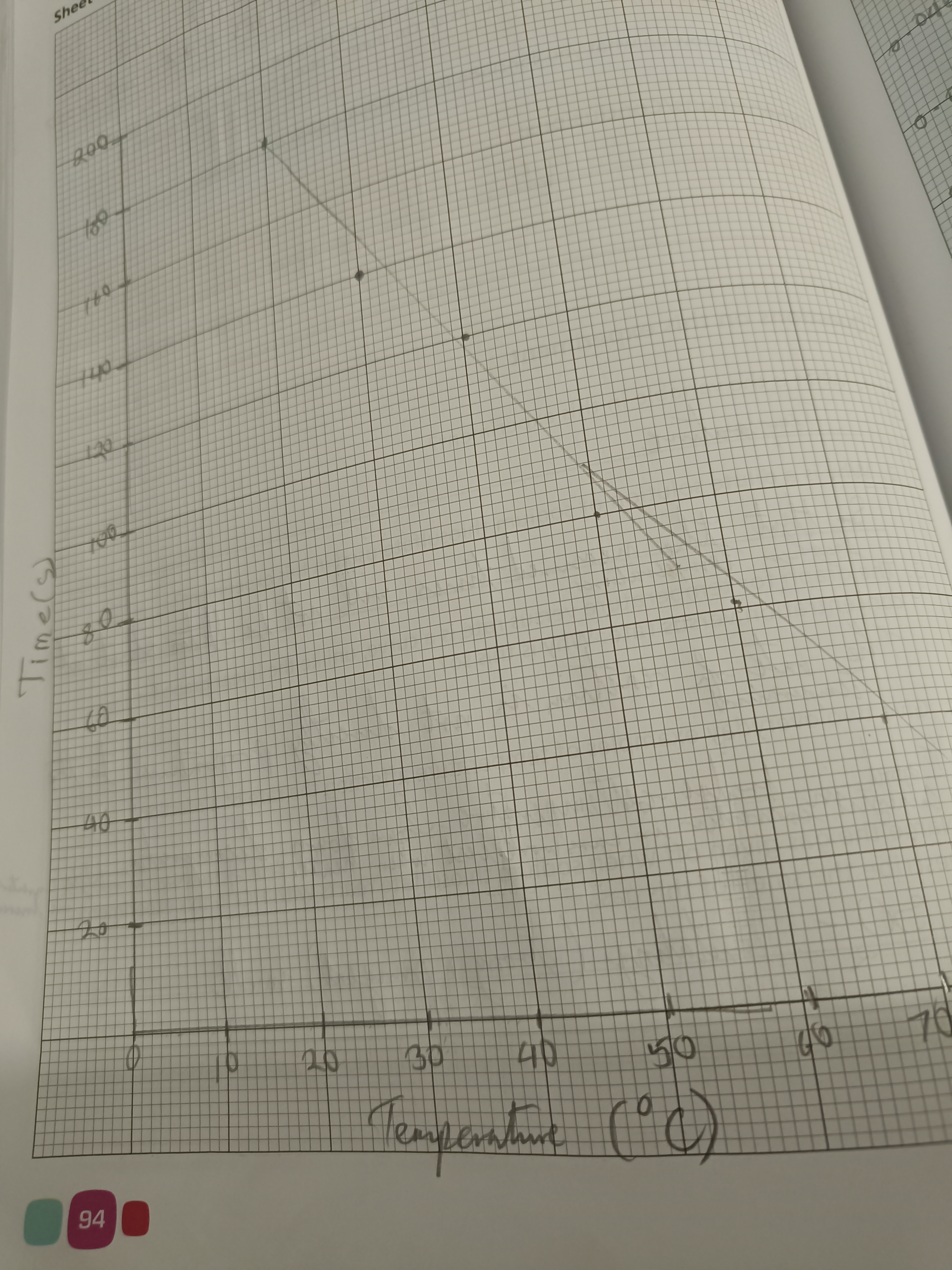
Draw a graph of time vs temp
…
Draw a graph of rate of reaction vs temperature
…