Unit 5: Acids & Bases CHEM
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
22 Terms
Buffered Solutions
>Contains a weak acid (HA) and its salt (NaA) or a weak base (B) and its salt (BHCl)
>Resists a change in its pH when H or OH- is added
>For a buffered solution containing HA and A-, use Henderson-Hasselbalch
> Capacity of a buffer depends on the amounts of HA and A-
> The most efficient buffering occurs when the [A-]/[HA] ratio is close to 1
> Buffering works because the amounts of HA (which reacts with added OH-)and A- (which reacts with added H+) are large enough that the [A ]/[HA] ratio does not change significantly when strong acids or bases are added
Strong acid - strong base titration
H+ + OH- --> H2O
equivalence point: pH=7
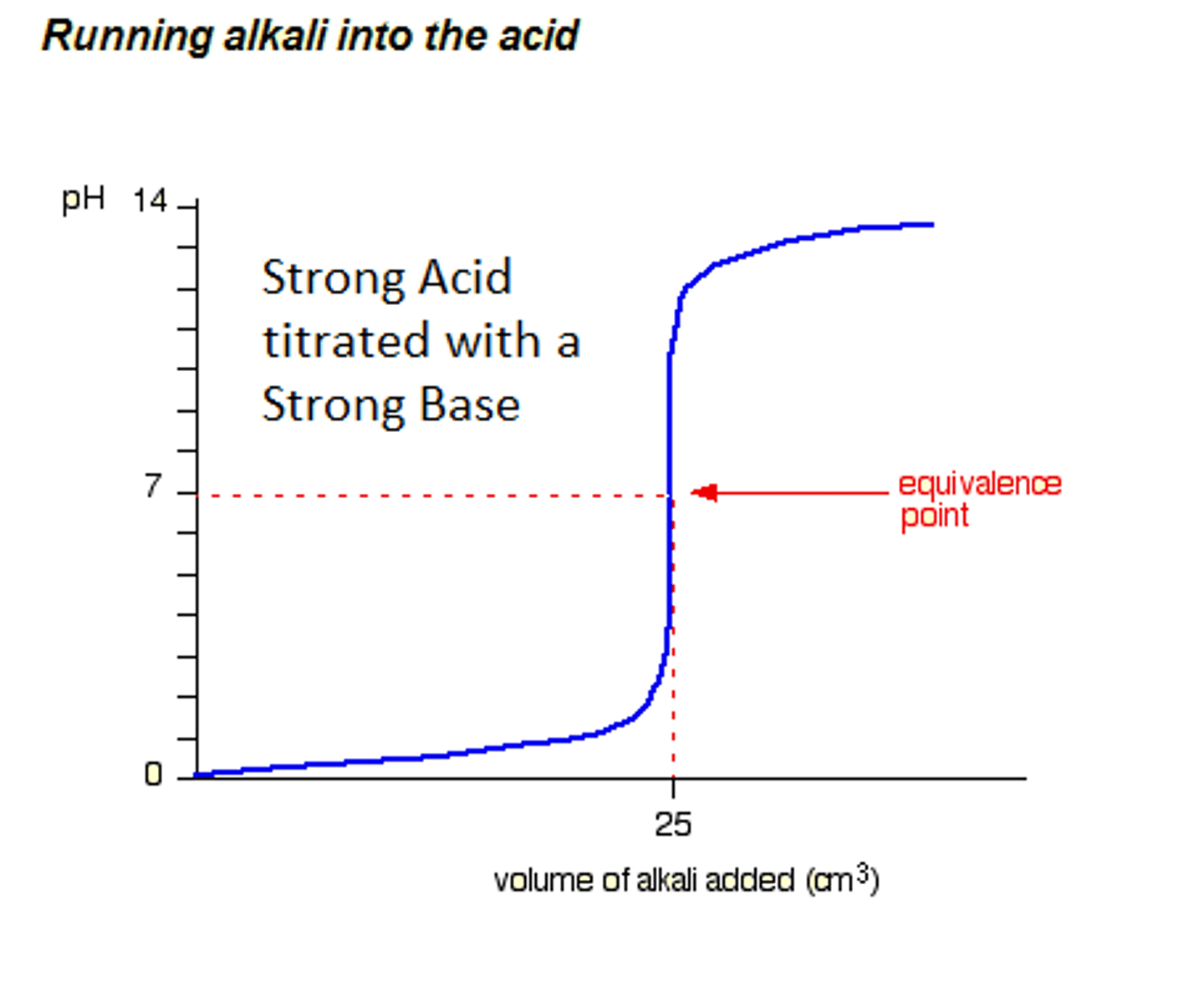
Strong base - strong acid titration
Equivalence point, pH = 7
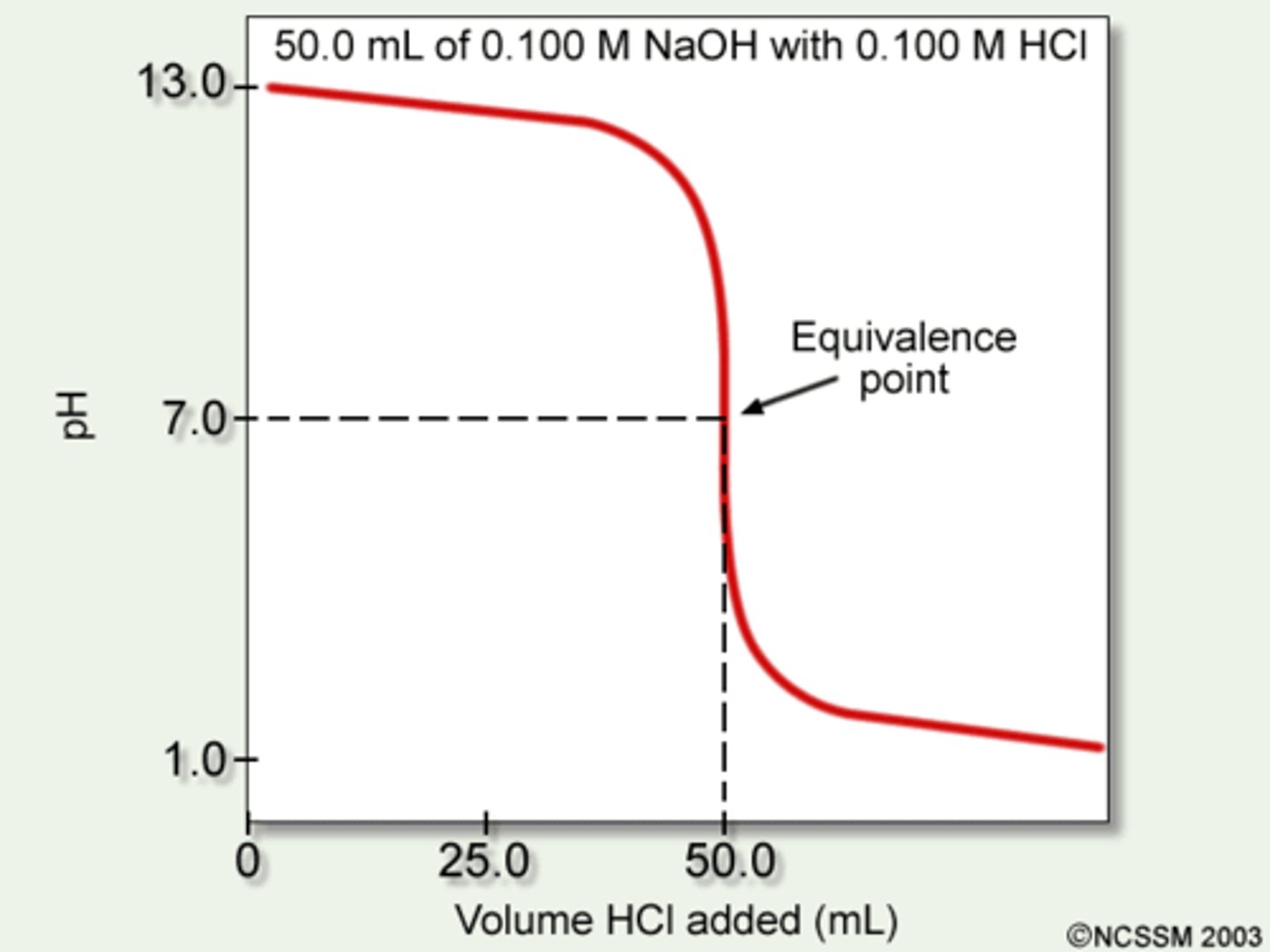
Weak Base - Strong Acid Titration
B + H+ --> BH+
equivalence point pH < 7
1. use stoich to calculate the new concentrations of B & BH+ after reaction
a) if no acid added, its a regular weak base calculation and use Kb = x^2/[B]
b) if some strong acid added, it's not enough to neutralize all of the weak base. Therefore, B and BH+ is left in the container. BUFFER! use the new moles after reaction and substitute it into Kb expression or HH equation
b1) halfway point: Half of B is neutralized, leaving equal molar amounts of Band BH+ , pKa = pH
c) equivalence point: all B reacts w/ added base H+ to make BH+. Now that only BH+ is left, this is a weak acid and produces an equilibrium BH+ H2O <-> B + H3O+ (flipped original eq). Divide by total volume and substitute [BH+] into Kb = x^2/M. The x is the [H+], which u can now use to find pH
d) Excess H+: If [H3O+] is in excess after sctoich (step 1), then divide by total volume to get new concentration and do pH stuff
![<p>B + H+ --> BH+</p><p>equivalence point pH < 7</p><p>1. use stoich to calculate the new concentrations of B & BH+ after reaction</p><p>a) if no acid added, its a regular weak base calculation and use Kb = x^2/[B]</p><p>b) if some strong acid added, it's not enough to neutralize all of the weak base. Therefore, B and BH+ is left in the container. BUFFER! use the new moles after reaction and substitute it into Kb expression or HH equation</p><p>b1) halfway point: Half of B is neutralized, leaving equal molar amounts of Band BH+ , pKa = pH</p><p>c) equivalence point: all B reacts w/ added base H+ to make BH+. Now that only BH+ is left, this is a weak acid and produces an equilibrium BH+ H2O <-> B + H3O+ (flipped original eq). Divide by total volume and substitute [BH+] into Kb = x^2/M. The x is the [H+], which u can now use to find pH</p><p>d) Excess H+: If [H3O+] is in excess after sctoich (step 1), then divide by total volume to get new concentration and do pH stuff</p>](https://knowt-user-attachments.s3.amazonaws.com/c3119c7a-8c5e-4204-a38c-dc956f899832.jpg)
Weak acid - Strong base titration
HA + OH- --> H2O + A-
equivalence point pH > 7
1. use stoich to calculate the new concentrations of HA & A- after reaction
a) if no base added, its a regular weak acid calculation and use Ka = x^2/[HA]
b) if some base added, it's not enough to neutralize all of the weak acid. Therefore, HA and A- is left in the container. BUFFER! use the new moles after reaction and substitute it into Ka expression or HH equation
b1) halfway point: Half of HA is neutralized, leaving equal molar amounts of HA and A- , pKa = pH
c) equivalence point: all HA reacts w/ added base OH- to make A-. Now that only A- is left, this is a weak base and produces an equilibrium A- + H2O <-> HA + OH (flipped original eq). Divide by total volume and substitute [A-] into Kb = x^2/M. The x is the [OH-], which u can now use to find pH
d) Excess OH-: If [OH-] is in excess after schoich (step 1), then divide by total volume to get new concentration and do pOH stuff
![<p>HA + OH- --> H2O + A-</p><p>equivalence point pH > 7</p><p>1. use stoich to calculate the new concentrations of HA & A- after reaction</p><p>a) if no base added, its a regular weak acid calculation and use Ka = x^2/[HA]</p><p>b) if some base added, it's not enough to neutralize all of the weak acid. Therefore, HA and A- is left in the container. BUFFER! use the new moles after reaction and substitute it into Ka expression or HH equation</p><p>b1) halfway point: Half of HA is neutralized, leaving equal molar amounts of HA and A- , pKa = pH</p><p>c) equivalence point: all HA reacts w/ added base OH- to make A-. Now that only A- is left, this is a weak base and produces an equilibrium A- + H2O <-> HA + OH (flipped original eq). Divide by total volume and substitute [A-] into Kb = x^2/M. The x is the [OH-], which u can now use to find pH</p><p>d) Excess OH-: If [OH-] is in excess after schoich (step 1), then divide by total volume to get new concentration and do pOH stuff</p>](https://knowt-user-attachments.s3.amazonaws.com/25de22ab-0784-4de5-afe7-73898c23f698.jpg)
When does an indicator change color?
when the less dominant form reaches 1/10 of the dominant form
Why do titration graphs end at the same point
A weak acid-strong base titration ends at the same point on the right as a strong acid-strong base titration bc the same molarity of titrant was used
rule: weak acids/bases & strong acids/bases take the SAME time to neutralize if the molarity of the titrant is the same.
titrant
A solution of known concentration that is used to titrate a solution of unknown concentration
in buret
analyte
Substance being analyzed
in Erlenmeyer flask
Best buffer
when [HA]=[A-]
pH = pKa
Best indicator for titration
pKa = 1 +- pH (changes color when pH is around there)
weak acid/base & strong acid/base in a solution
the strong acid/base dominates, thus you can ignore the concentration of weak acid/base
in a weak acid-strong base titration, where does pH only depend on [HA]?
where does it only depend on [A-]?
depends on [HA] before the equivalence pt.
depends on [A-] at the equivalence pt and after it (at eq pt, only A- is present bc all HA reacted w/ OH-, producing H2O and A-)
autoionization of water
when pure water reacts with itself to form, hydronium and hydroxide ions
since the autoionization of water is endothermic, increasing heat would make it shift right. the amount of H+ and OH- would increase. As a result, pH & pOH would decrease but it would stay neutral.
how to know if something is an acid, base or neutral
Test H2O with each ion
put the cation w/ OH
put the anion w/ H+
if weak base and strong acid, its a strong acid
strong base vs conjugate acid
strong base = weak conjugate acid
strong acid = weak conjugate base
diluting an acid with water
moves the pH up towards 7
(can't surpass 7)
[H+] decreases as volume increases
You're given a pH graph showing pH after adding an increasing volume of titrant. You're given the volume of the analyte and the molarity of the titrant. How do you find the concentration of the analyte?
How do you get the Kb/Ka value?
Look at the equivalence point to find the volume of titrant used. Convert this to moles. Because it's equivalence, the moles of titrant and analyte must be equal, so the number you got is the # of moles of analyte. Divide this by the initial volume of the analyte to get molarity.
Look at the halfway point, where pH = pKa. Convert this to Ka and then get what you're looking for.
Choosing an indicator
An indicator's pKa should be +- 1 the pH at the equivalence point
Buffer range & capacity
Buffer range: Bufferts that contain equimolar quantities of weak acid and conj. base (ideal buffer) has a usuable pH range within +- pH unit
the higher the concentrations of acid & conj. base, the better the buffer bc higher capacity
Capacity: How much acid/base can it neutralize before the buffer is exhausted
Henderson-Hasselbalch equation
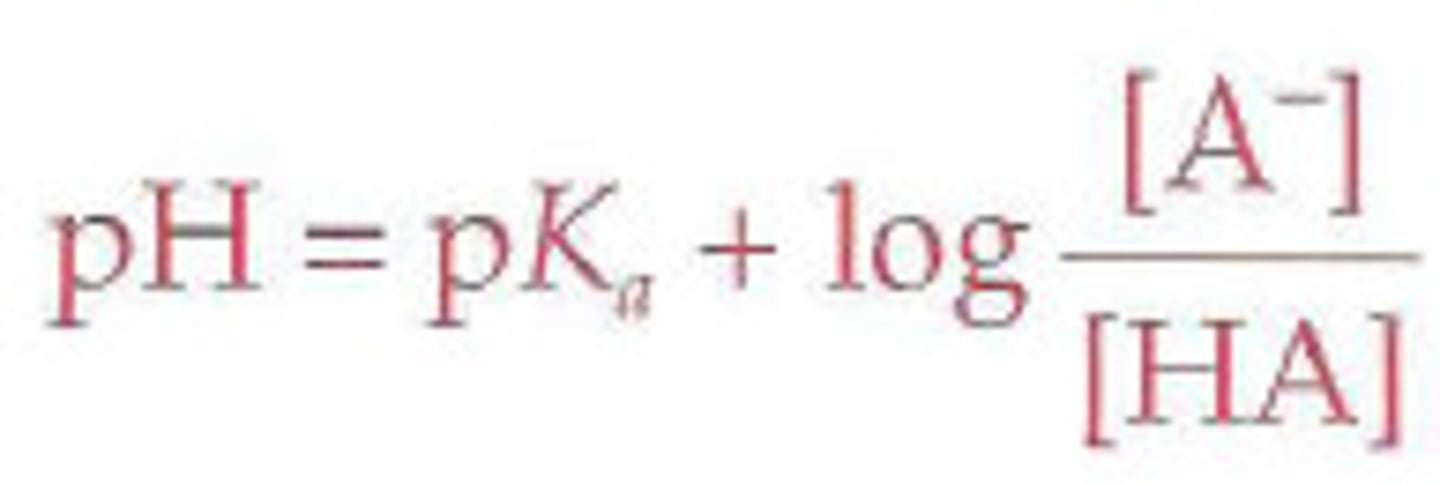
given a weak acid and asked to write its dissociation. So it dissociates into
HA + H2O --> H3O + A-
then it says "you add NaOH" to it
this wouldn't react w/ H+ , it would react with HA. that's the point of a buffer