Bacterial diseases
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
22 Terms
1. Mycobacteriosis,tuberculoses
TUBERCULOSIS
Caused by:
Mycobacterium bovis – low host specificity, chronic disease in warm blooded animals
M. avium – generally affects birds, occasionally ruminants and pigs
M. acvium subsp. Avium
M. tuberculosis – most host specific. Respiratory tuberculosis of humans and non- human primates, occasionally pigs, dogs and birds.
Susceptible species: Group of contagious zoonotic diseases affecting domestic animals, wildlife and humans. OIE notifiable! Zoonotic!
Transmission: M. bovis found in respiratory secretions, exudates from lesions, urine, feces, milk, vaginal secretions and semen. Close contact, inhalation (most common in cattle) or digestion.
Clinical signs:
CS can take multiple months to develop, can remain latent for years.
Cattle – usually chronic:
- Weight loss, emaciation, weakness, anorexia
- Fluctuating fever, lymphadenopathy
- Intermittent cough, dyspnea
Birds – the primary lesion is almost always in the intestinal tract.
- Form deep ulcers filled with caseous material containing mycobacterial cells.
- Usually no clinical signs, chronic and progressive wasting and weakness
- Diarrhea is common
Pigs typically have no clinical signs, lesions found during meat inspection after slaughter
Pathology:
Mainly affect the lungs and liver. Cause formation of granulomas, where bacteria hide, they undergo necrosis in the center. If bacteria gain entry to blood stream they can spread throughout the body and set up many foci of infection. This may be generalized and rapidly fatal, for example in miliary tuberculosis or pearl disease.
In many cases the tissue destruction and necrosis is often balanced by healing and fibrosis, and affected tissue is replaced by scarring, and cavities filled with caseous necrotic material.
● Respiratory tuberculosis: bronchopneumonia with chronic wet cough, dyspnoea and tachypnoea.
The lesions may be found by percussion or auscultation of the respiratory system.
● Miliary tuberculosis: nodules on several organs
● Pearls disease: multiple nodules on pleura and peritoneum (serosal surfaces)
Diagnosis, Treatments and preventative:
Tuberculin test, ELISA
Quarantine, herd protection, slaughterhouse inspection, pasteurization of milk, elimination of infected animals. No vaccine.
Treatment: can try ATB

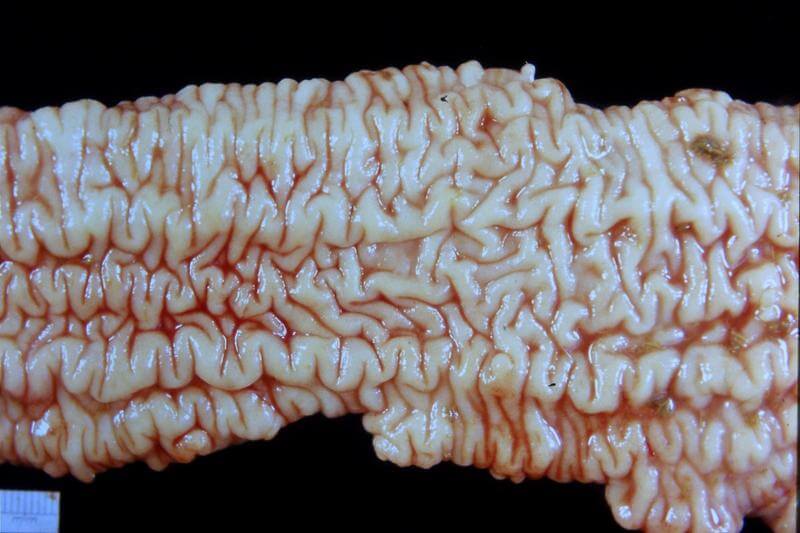
2. Paratuberculosis
Caused by:
M. avium subsp. Paratuberculosis – John’s disease (similar to Chron`s in humans). OiE-notifiable.
Susceptible species:
Paratuberculosis (Johne’s disease) is a chronic, contagious bacterial disease of the intestinal tract that affects mainly sheep and cattle, as well as other ruminants species. It has been reported in other mammals
Transmission:
Infected animals sheds the bacteria in manure, colostrum and milk.
Ingestion: infection is most commonly acquired in young animals through contamination of the environment or through ingestion of contaminated milk.
Vertically: the disease can also be transmitted from an infected animal to its foetus.
Epizootology:
Not a zoonosis, however, is very similar to Chron’s disease in humans. The bacteria has a global distribution and is very resistant to both heat, cold, and drying.
Adult animals are less likely to be infected than young animals (highly susceptible).
Clinical signs:
Paratuberculosis is a slowly progressive disease, and clinical signs usually first appear in young
adulthood (4-7 years old).
Predisposing factors include stress and poor nutrition.
The bacteria cause chronic hypertrophic
enteritis characterized by diarrhoea (very watery
and smelly - look like pea soup), unthrifty
animals, low milk yield and progressive weight
loss despite a good appetite and normal body temperature.It may also cause what is known as “bottle jaw”- swelling under the jaw.
The symptoms become gradually more severe and lead to malnutrition and death.
Pathology:
The primary site of infection is the Ileum.
The wall of ileum contains Peyer’s patches containing macrophages which engulf M. paratuberculosis, but fails to kill it.
Inside the macrophage, M. paratuberculosis multiplies until it kills the cell and infect other cells.
The animal’s immune system reacts to the bacterial invasion by recruiting more macrophages and lymphocytes.
Infiltration of infected tissues with millions of these cells leads to visible thickening of the intestines.
This prevents nutrient absorption and diarrhoea results.
Diagnosis, prevention, treatment:
Clinical signs, Laboratory tests: faeces, PCR, allergy test, Biopsy
There is no known treatment for the disease.
Control involves good sanitation and management practices including screening tests and surveillance
Brucelloses
Caused by:
B. melitensis – sheep, goats, cattle, dog, humans
B. suis – wild boars, wild hares, dog, human
B. abortus – cattle, horses, sheep, goats, dogs, human
B. canis – dog, man
B. ovis – sheep
Susceptible species:
- Zoonotic!
B. melitensis, B. suis, B. abortus, B. canis can also infect humans → Malta fever!
Transmission:
Virus is shed in aborted fetus, membranes, uterus discharge, semen, milk, oral, sexual contact, damaged skin, conjuctiva.
Infection by ingestion or direct contact with birth products, or by unpasteurized milk or undercooked meat in humans.
Epizootology:
IP: variable, 21-200 days
Clinical signs:
1. Cattle (B. abortus, B. melitensis, B. suis)
Abortion (6-8th month), retention, mucopurulent discharge, orchoepidimytitis, arthritis, bursitis, hydroma (swollen joints), abscesses
Horses (B. abortus, B. suis, B. melitensis)
Arthritis, tenditis, osteomyelitis, sternal abscess
Swine (B. suis)
Abortion, orchoepididymitis, subcutaneous abscess, spondylitis, paresis, paralysis
Sheep and goats (B. melitensis, B. abortus, B. ovi)
Abortions (3-5 months), orchitis, epidymitis, arthritis
Humans
- Undulant fever (Bang fever), spondylitis, swollen joints, orchitis, myalgia, epidimyditis, hepatosplenomegalia, lymphadenitis
Pathology:
After entering the body, phagocytosis, but not killing, persistence in mononuclear cells.
Brucella forms granulomatous nodules in which intracellular growth is favored (3 days – 3 months)
Diagnosis:
Demonstration of brucella organisms by staining methods, culture of specimens, isolation of brucella by animal inoculation.
Serological methods:
- Complement fixation test
- Tube agglutination
- Slide agglutination – RBT (rose Bengal test)
- ELISA
- Milk ring test
Prevention and treatment:
No treatment. Some vaccines are available. Selecting brucella free animals for breeding, quarantine, remove and destroy placenta.

Listeriosis
Caused by:
Listeria monocytogenes (most common)
L. ivanovii
Susceptible species:
Mammals, birds, reptiles, amphibians and fish.
Rodents are reservoirs!
Most often seen in cattle, sheep and goat.
ZOONOTIC
Transmission:
Mainly ingestion, from soil, plants, water,
often linked to eating silage in cattle.
For humans it can be unpasteurized milk and undercooked meat, direct contact with placenta and fetus.
Can be shed in feces of infected animal.
Epizootology:
Very resistant to environmental changes
Replication is stopped at under 2 C and above 45C.
Pasteurization inactivates bacteria at 72C.
Refrigerator temp and frozen food are favorable.
Occurrence in soil (at 5C for 5 years), plants, animals, feces and water (1-2 years) and silage (12-16 months).
Seasonal incidence, sporadic or enzootic during winter-spring.
Predisposing factors: hygiene, nutrition, and infectious or non-infectious diseases.
Asymptomatic carriage is more common.
Clinical signs:
Reproductive losses are one of the most common signs. May abort late in gestation or give birth to stillborn offspring. Cause retained placenta, metritis and septicemia.
CNS disease, in adults. Depression, anorexia, facial paralysis, dysphagia, excessive salivation, nystagmus, incoordination. Paralysis of limbs, animals cannot move, typical are swimming movements → coma, death after 3-10 days.
Septicemia is seen in young ruminants or adults with metritis. Gastroenteritis, weakness, anorexia, serous eye discharge, death. Most common form in birds.
Clinical signs in humans:
- Reproductive losses: abortion, stillbirth, fever, headache. Symptoms occur in 3rd trimester.
- Septicemia
- CNS disease: encephalitis, meningitis, seizures can occur.
- Febrile gastroenteritis: diarrhea, fever, nausea, headache “flue-like symptoms”.
- Skin rashes (rare, may occur in veterinarians)
Pathology:
Alimentary route of transmission, from contaminated food, or through conjuctiva and urogenital system.
Entry by blood and lymphatic circulation to parenchymatous organs, CNS and genital tract.
Migration of bacteria along peripheral nerves to CNS.
In pregnancy, transfer from genital organs through placenta to fetal fluids which are aspirated by fetus and cause generalized infection → abortion, stillbirth
Septicemic form – haemorragies in pleura, epicardium, necrotic lesions in liver and spleen.
Encephalitic form –oedema of brain, suppurative meningoencephalitis
At abortion we can observe changes in chorioplacental – necrotic lesions, aborted foetus is oedematous, mummified, spleen is enlarged, necrotic lesions in liver
Diagnosis:
Clinical signs, necropsy, microbiological tests, agent isolation, serology (ELISA and PCR)
Sampling: heart, liver, kidneys, spleen, brain, blood, CSF
Prevention: ensure optimum conditions for breeding and nutrition, correct process of silage and hay fermentation, hygienic standards of silage and hay, control of fodder, regular disinfection and rodent control.
Therapy and control: ATB, vaccination in sheep.

Tularemia
Tularemia (rabbit fever disease)
Caused by:
Franciscella tularensis
Susceptible species:
Rabbits and other wild rodents primarily.
It can also affect livestock animals, sheep especially.
Transmission:
By blood-sucking parasites such as ticks and flies.
Humans and animals can be infected by ingestion, inhalation, direct contact with infected animals and environment, by blood sucking parasites or biting, scratching by dogs and cats. - ZOONOTIC
Rodents, rabbits and hares are reservoirs!
Epizootology:
The bacterium has several subspecies with varying degrees of virulence.
F. tularensis tularensis (type A), found in lagomorphes in North America, highly virulent in humans and domestic rabbits
F. tularensis palaearctica (type B) occurs mainly in aquatic rodents, less virulent for humans and rabbits.
Clincial signs:
Highly susceptible and highly sensitive species: severe course, septicaemia, high lethality, death within 5 -12 days: fieldmouse, water-rat, hamster, brown hare,
Low susceptibility and sensitivity species: mild, inapparent course of infection: foxes, dogs, cats, cattle, sheep, horses
Clinical signs in animals:
subclinical infections
moderate to very high fever
face and eyes redden and become inflamed
inflammation spreads to lymph nodes, which enlarge and may suppurate
after transmission by parasites 2-3 days: septicaemia
→ fever, lethargy, anorexia, signs of septicemia and possibly death
in 4-13 days death
chronic diseases 14-60 days death.
Sheep: rhinitis, conjunctivitis, paresis
Cattle: inapparent course, abortion
Pigs: cough, rapid respiration
Dogs: loss of appetite, mild fever,
Cats: high fever, lymphadenopathy
Clinical signs in humans:
Systemic/internal form: after penetration of bacteria by inhalation or when pathogen reach internal organs by blood
→ Thoracic form: lungs – pneumonia, cough, dyspnoe, pain in thorax
→ Abdominal form: typhus-like disease, swollen liver and spleen,
abdominal pain, diarrhoea
External form
→ Ulceroglandular, oculoglandular, oralglandular
→ On the site of entry: red painful nodule – ulcer
→ Corresponding lymph nodes are swollen, painful, purulent
→ Fever or no fever
Pathology
Primarily infects macrophages.
The course of disease involves spread of the organism to multiple organ systems, including the lungs, liver, spleen and lymphatic system.
Exact cause of death is unclear, thought to be a combination of multiple organ system failures.
Diagnosis
Agent identification, PCR, ELISA
Therapy:
antibiotics (streptomycin, tetracyclin, erythromycin..) resistance to penicillin and sulphonamids
Control:
professional risk – agriculture workers and laboratory staff vaccination.
After recovery, immunity for years.

Leptospirosis
Caused by:
Leptospira interrogans, with serotypes; canicola (dogs are reservoir hosts), grippotyphosa, hardjo, icterohaemorrhgiae, Pomona (pigs)
L. biflexa
Susceptible species:
Animals and humans, zoonotic!
Humans are considered incidental hosts.
Mainly dogs, cattle, sheep, goats, horses and pigs.
TRANSMISSION:
Directly between hosts, by the skin
Indirectly through environment: shed in the urine of infected animals, including rodents and domesticated animals, which may not show signs of disease.
Humans usually become ill after contact with infected urine, or through contact with water, soil or food that has been contaminated.
Epizootology:
Worldwide distribution
Clinical signs:
Often related to kidney and liver disease, or reproductive dysfunction.
In humans, many cases are asymptomatic.
IP: 5-15 days
Dogs:
→ Sudden fever
→ Stiffness in muscles, legs, and stiff gait
→ Shivering, weakness, depression, lack of appetite
→ Increased thirst and urination, rapid dehydration,
→vomiting, diarrhea
→Icterus and anemic symptoms
Cattle:
→ Acute form can be severe in calves; fever, anorexia, dyspnoe, icterus, hemoglobinuria, hemolytic anemia
→ Chronic form; manifest as abortion 6-12 weeks after insemination, and stillbirth
Horses:
→ Uveitis or abortions,
→ Mild fever, anorexia, hemolysis, anemia, icterus, depression
Pigs
→ abortion
Pathology:
Acute renal failure occurs in 80-90% of dogs, icterus seen in post-mortem examination
Penetrate mucous membranes and skin → rapid replication in blood → vasculitis → multiorgan infection → production of toxins.
Diagnostic:
Clinical signs, combination of serology to detect antibodies and PCR to detect the organism, as most animals are vaccinated.
Microscopic agglutination test (MAT) - most used serological test, agglutinated if presence of antibodies.
Identification of the agent
→ Post mortem- of internal organs,
→ In body fluids
→ Microscopic examination, histology and immunofluorescence
Treatment and prevention:
ATB, fluid therapy, blood transfusion and supportive care.
Inactivation by temperature, UV, disinfection and freezing.
Prevention by vaccination, rodent control and contact with reservoir host.

7. Spirochaetosis
Pathogenic members of spirochetes:
Leptospira → leptospirosis
Borelia → lyme disease
Treponema → syphilis
Brachyspira → intestinal spirochaetosis
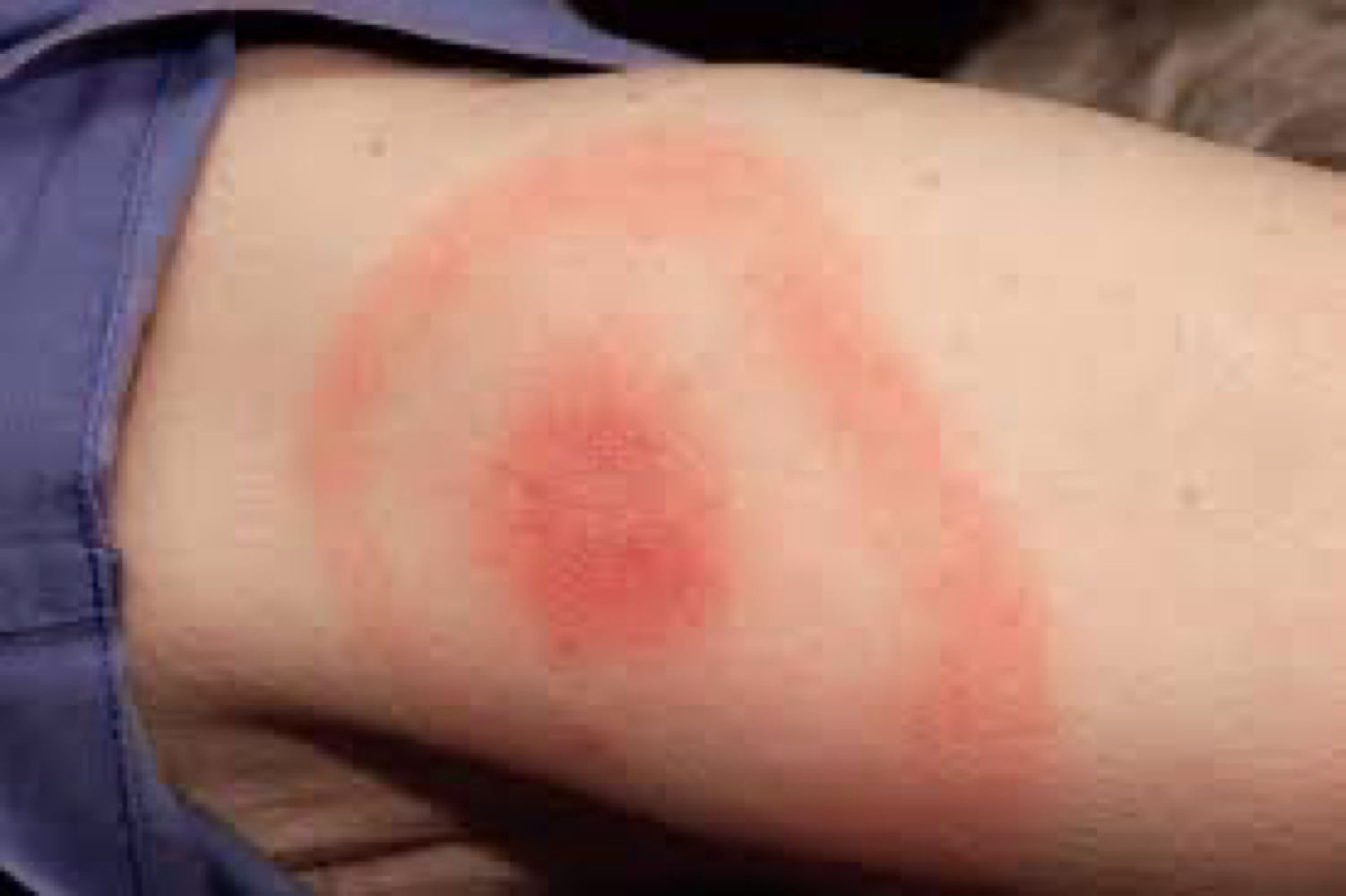
Lyme disease
Caused by:
Borelia burgdorferi
B. garinii (birds)
Susceptible species:
Mammals, birds, and reptiles serves as reservoirs.
Zoonotic!
Transmission:
through ticks (genus Ixodes)
Epizootology:
Rodents, insectivores, and other small mammals are the main reservoirs.
Clinical signs:
Most infections in animals are asymptomatic.
Dogs + generally:
→ Arthritis, lameness
→ Non-specific signs; fever, anorexia, lethargy, lymphadenitis
Horses:
→ Uveitis
→ Blindness and neurological signs
Humans
→ 1st stage is influenza-like symptoms
→ 2nd stage is Erythema migrans (rash) (Erythema Chronicum Migrans) den Røde ringen!!
→ 3rd stage is arthritis and CNS symptoms
Diagnosis, Prevention, treatment:
Clinical signs, endmic area of ticks
ELISA, PCR, bacterial culture
ATB.
Vector control – tick repellents, vaccination
Syphillis
Caused by: Treponema cuniculi
Susceptible species: Rabbits
Transmission: Sexually, but also maybe through milk from infected doe to offspring.
Clinical signs:
In some rabbits, the bacterium may remain dormant for long periods of time, even years, and the rabbit may not show any clinical signs until a stressful event occurs.
Affect mucocutaneus junctions of genitalia, anus and/or the face.
Crusty and ulcerated skin
Pus-like exudate and bleeding
Diagnosis and treatment:
biopsy
ATB
others:
Swine dystentery – brachyspira hyodysenteriae
Swine colitis – brachyspira pilosicoli
Staphylococcosis
Staphylococcosis is any infection or disease caused by members of the genus staphylococcus.
S. aureus – local skin disease and systemic diseases
S. hyicus – exudative epidermittis of swine
S. intermedius - dogs
Most staphylococcus are harmless and is a part of normal skin microflora, may enter through cuts, can spread in the body and produce toxins like hemolysin, enterotoxin and various enzymes
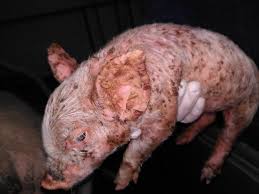
Exudative epidermitis of pigs
Caused by: Staphylococcus hyicus
Susceptible species: Pigs
Transmission:
Spread by contact and typically enters skin wounds or by parasites.
Very contagious.
Clinical signs:
Peracute:
→ Affect sucklings
→ Erythema, pain, anorexia, dehydration
→ Greasy exudate from eyes, ears and abdomen
→ Death within 48h
→ No pruritus!!
Acute:
→ similar to peracute + skin thickening and crusting.
→ Death within 4-8 days.
Chronic: sporadic, 50-70% mortality
Diagnosis:
Skin scrapings and biopsy.
Many diff. dg like parakeratosis, scabies, pox and dermatomycosis.
Prevention and treatment:
Isolation of youngs from sows, cleaning and disinfection,
ATB (less effective in animals up to 10 days)
No vaccine!
Tick pyemia of lambs
Caused by: Staphylococcus aureus
Susceptible species: Sheep
Epizootology:
Occurrence in area with ticks, ixodes Ricinus.
Seasonal incidence.
Predisposition factor: tickborne fever caused by Anaplasma phaocytophilum → immunosuppression → higher susceptibility to S. aureus.
Clinical signs:
Septicemia, sudden death
Local infection, arthritis, meningitis
Crippling, lameness, paralysis
Abscesses in internal organs, joints and meninges
Diagnosis, treatment:
Skin sample
Tick control, preventative ATB application in 1-3w and 5-7w of life.
If clinical signs appear, treatment is not effective.
Staphylococcosis of dogs and cats
Staphylococcus intermedius (dogs)
S. felis, S. simulans (cats)
Dogs and cats.
Sporadic in immunosuppressed individuals.
Transmission: Through damaged skin and mucosa.
Clinical signs:
→ A purulent exudative inflammation in skin, ears, eyes, respiratory and urogenital system.
→ Skin abscesses
→ Otitis, pyoderma
→ Endocarditis, bronchopneumonia, UGT
Diagnosis, treatment:
Bacteriology, skin scraping, ATB
Streptococcosis
Streptococcosis are any diseases caused by the bacteria Streptococcus. Species are classified based in their hemolytic properties:
Alpha-hemolytic species cause oxidization of iron in hemoglobin
Beta-hemolytic species cause complete rupture of RBC
Gamma-hemolytic species cause no hemolysis
Streptococcal meningitis of pigs
Caused by: Streptococcus Suis type 2
Susceptible species:
Affect swine (10-14 days post weaning), cattle, sheep, goats and humans. Zoonotic!
Transmission:
Source of infection is from healthy carriers (present in tonsils, nasal mucosa, vaginal secretions)
ingestion, inhalation or nose-to-nose contact, and from mother to young.
Stress increase the risk (thus during weaning)
The bacteria can reside in tonsils for more than 1 year.
Epizootology:
IP: 24h-2weeks
Clinical signs:
Young animals: septicemia, arthritis
Older animals: meningitis, endocarditis
Sudden death of several pigs
Fever, anorexia, depression, tremor, ataxia, convulsions, blindness
Lameness, abscesses
Diagnosis
Isolation of bacteria from CSF, brain, lungs, synovial fluids, heart
Prevention and treatment:
good hygiene, quarantine, ATB, vaccination not effective
Other diseases:
Strangles
Mastitis (S. agalactiae, S. dysgalactiae)
Avian streptococcosis
Streptococcosis of dogs and cats
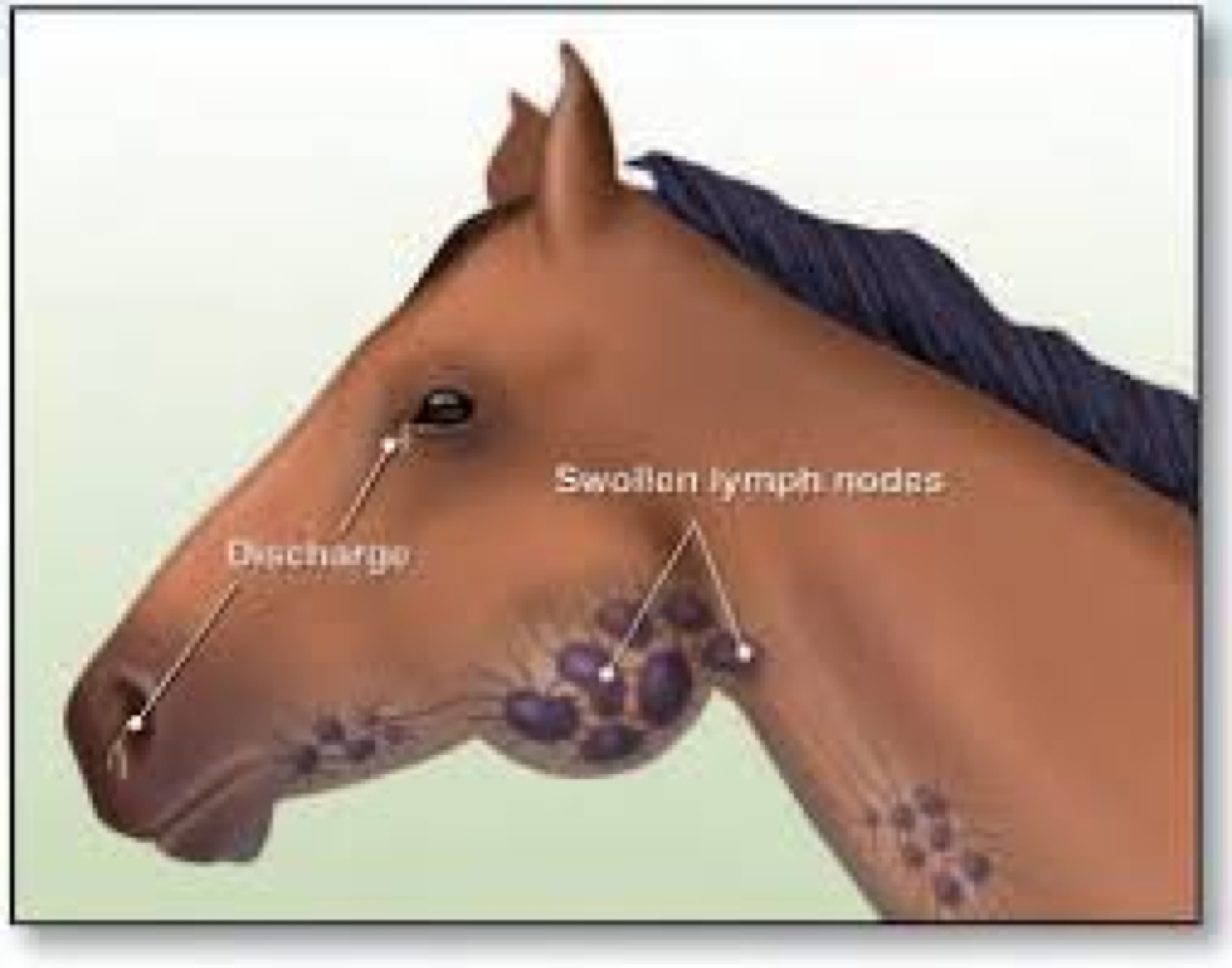
Strangles
Caused by:
Streptococcus equi subsp. Equi
Susceptible species: Horse, 5 months to 5 years
Transmission:
Infected horses are source of infection
spread by direct contact, aerosol, feed, water, equipment.
Epizootology:
Recovered horses may shed bacteria from their nose and saliva for up to 6 weeks following infection.
All horses that have been infected with equine strangles should be isolated from other susceptible animals for a minimum of 6 weeks following infection
Clinical signs:
IP: 1-3 weeks
Purulent inflammation of upper respiratory system, pharynx, regional lymph nodes
Acute rhinitis, pharyngitis, laryngitis and regional purulent lymphadenitis
Dissemination to other LN and organs; lungs, brain, liver, spleen and joints
Abscess formation
Fever, anorexia, depression, nasal discharge
Dyspnoe, reproductive cough, swallowing difficulties
Enlarged, painful, hot lymph nodes
Lymphadenitis, purulent nodules
Obstruction edema of legs
Complications; pneumonia, meningitis, peritonitis
Atypical form: mild fever, anorexia, nasal discharge
Laryngeal hemiplegia; involves paralysis of throat muscles, commonly referred to as roaring. May follow abscessation of cervical LN
Anemia
Guttural pouch emphyema – filled with pus, which may be concurrent with classic strangles, or follow in the immediate convalescent period
After clinical recovery; purpura hemorrhagica, an immune-mediated complication, 30-50% mortality!
Diagnosis:
Clinical signs, bacteria isolation from nasal swabs and pus, → Ag detection by hemoagglutination
Prevention and treatment:
Quarantine and good hygiene.
Treatment with ATB, vaccination, surgery
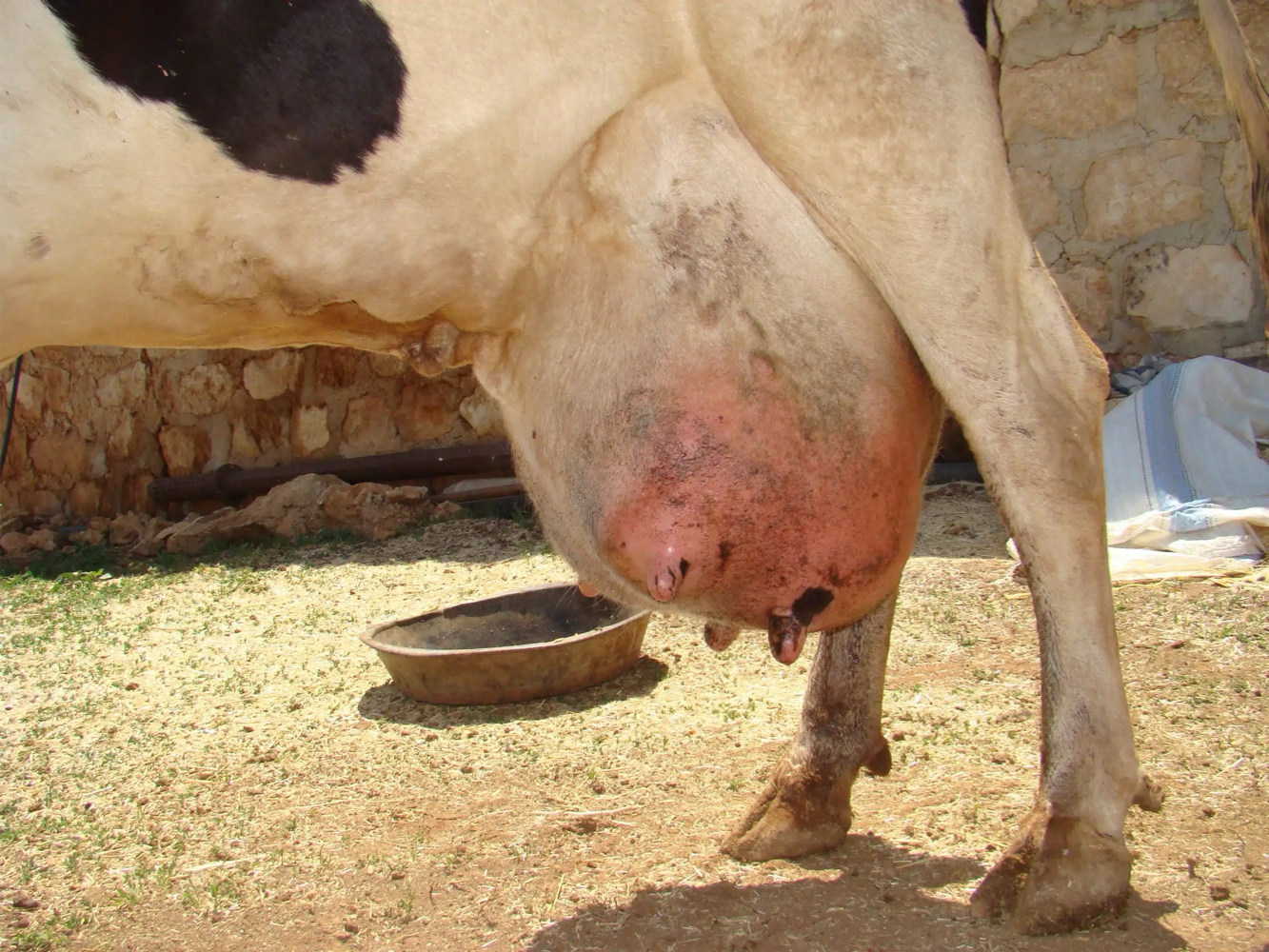
Mastitis
Mastitis is the persistent, inflammatory reaction of the mammary gland due to physical trauma or microorganisms’ infections.
Most common disease in dairy cattle, characterized by physical, chemical and bacteriological changes in the milk and pathological changes in glandular tissue.
There exists several form based on duration – acute, subacute, chronic, subclinical, latent.
Caused by:
Almost any microbe that can opportunistically invade tissue and cause infection can cause mastitis.
Most infections are caused by streptococci, staphylococci and gram-negative rod species.
2 types of etiological agents, based on origin and transmission methods: Environmental and Contagious
Environmental – infected from the environment
→ Streptococcus dysgalatiae
→ Streptococcus uberis
→ Streptococcus bovis
→ E. coli
Contagious – indirectly or directly via milking equipment, humans hands ect.
→ Streptococcus agalactiae
→ Staphylococcus aureus
→ Corynebacterium bovis
→ Mycoplasma bovis
Susecptible species:
All animals with mammary glands, but most common in dairy cattle.
Predisposing factors: lack of milking hygiene and general hygiene, abnormal shape of teat, lesions on teat, immunosuppression and in the first 2 months of lactation.
Clinical signs:
Clinical mastitis:
Usually caused by environmental pathogens!
Local signs include changes in size, secretions (presence of flakes or clots), consistency and/or temperature of mammary glands.
Systemic signs include fever, tachycardia, depression, loss of appetite and dehydration.
→ Peracute mastitis show all signs of local inflammation, as well as severe systemic signs
→ Acute mastitis show all signs of local inflammation, with less severe systemic signs
→ Chronic mastitis show minimal changes in the milk, the gland is hard at palpation
Subclinical mastitis:
No clinical signs are present.
Disease is recognized by increased somatic cell count indicating udder inflammation, positive bacteriology, and decreased milk production.
Pathogenesis:
3 phases:
● Invasion phase: organism passes from exterior into the teat canal.
● Infection phase: organisms multiply and invade the mammary tissue.
● Inflammation phase: appearance of clinical mastitis or greatly increased somatic cell count.
Diagnosis:
Local clinical signs, palpation and inspection of the udder
Examination of tile milk
→ pH, more alkaline
→ California mastitis test
→ Somatic cell count (above 300 000cells/ml → mastitis)
→ Bacteriological examination – isolation and identification of spp.
CAMP-test → Detect Streptococcus agalactica (positve when arrow is formed)
Treatments and preventative:
ATB - systemic and/or intramammary
Improve milking hygiene, teat dipping, develop program to prevent the spread of bacteria at milking time.
Eliminate existing infections by treating all cows at drying off and culling chronic cows
Salmonellosis
Caused by and susceptible species:
Salmonella enterica,
S. bongori.
Family Enterobacteriae
S. enterica most important subspecies is enterica, which we can further divide into 2 main groups with subspecies:
Typhoidal: salmonella enterica subsp. enterica serovar typhi (only humans)
Non-typhoidal
S. enterica subsp. enterica divided into following serovars:
→ S. enteritidis – horse, poultry
→ S. paratyphi – humans
→ S. typhimurirum – cattle, swine, horse, humans, poultry, sheep, rodents
→ S. cholerasuis – swine
→ S. Dublin – cattle
Transmission:
Oral route, usually through contaminated feed and water (milk and meat in humans).
Birds may serve as vectors.
The bacteria may survive for months in wet, warm areas such as pig barns of water dugouts.
Leading cause of foodborne diseases in humans
Clinical signs:
Generally presents as enteritis and septicemia in most animals.
Most animals are carriers, and they don’t have any clinical signs.
Clinical disease typically occurs in young, pregnant, and lactating animals and during stress.
Enteritis – ruminants, pigs and horses
Diarrhea, dehydration, depression
Abdominal pain, anorexia, fever
Decreased milk production
Death from dehydration and toxemia
→ Subacute; adults, diarrhea, weight loss
→ Chronic; emaciation, fever, inappetence
Septicemia – ruminants, horses and pigs
Affect young animals
Depression, fever
CNS signs or pneumonia
Dark skin coloration
Death within 1-2 days
Other signs can be abortion, joint infections
Dogs and cats – acute diarrhea, septicemia, abortion
Birds – very young birds, anorexia, lethargy, diarrhea, CNS signs
Pathogenesis:
After ingestion, the bacteria multiply in the intestine causing enteritis.
It may invade the blood stream and cause further infection in brain, meninges, pregnant uterus and bones.
Diagnosis:
Bacterial cultivation from feces, ELISA, PCR
Treatment, prevention:
ATB, fluids and NSAIDs
Good hygiene, buy salmonella-free animals, all in – all out, minimize stressful events

Fowl typhoid - Pullorum disease
Caused by:
2 different biovars of Salmonella enterica subsp. enterica → biovar pullorum and biovar gallinarum
Susceptible species: Birds
Transmission:
Orally and via the respiratory tract.
Found in feces of birds.
Vertical transmission in pullorum disease!
Clinical signs:
Pullorum disease is usually symptomatic only in young birds
Fowl typhoid also affects growing and adult poultry
Pullorum:
birds at 3-4 weeks old
Dead and dying chicks may be found after hatching - High mortality.
White diarrhea, seen around the anus
Non-specific signs of acute septicemia; depression, weakness, loss of appetite, huddling, dehydration, ruffled feathers, diarrhea
Die of acute septicemia, may be no lesions.
Less acute in older chicks, inapparent in older than 4 weeks
Arthritis
Fowl typhoid:
Affect all ages
Similar clinical signs to pullorum.
Older birds may be pale, dehydrated and have diarrhea.
Diagnosis:
Since clinical signs is very similar, diagnosis should be performed by isolation and identification of bacteria, necropsy, ELISA, PCR
Treatment and prevention:
ATB, good biosecurity, quarantine

14. Colibacillosis
Caued by: Colibacillosis refers to any infection or disease caused by the bacteria Escherichia coli.
Susceptible species:
Commonly found in lower intestine of warm-blooded animals.
Epizootology:
Not all E. coli are pathogenic, but some strains have developed virulence factors and can release toxins; resistance to phagocytosis and can adhere to host structures.
Transmission:
Fecal-oral route.
Sick, immunocompromised animals or animals in a dirty environment are predisposed.
Pathogenesis:
The relationship between the host intestine and bacteria is usually symbiotic.
If there is any change in bacteria or host, E.coli can become severely pathogenic – immunosuppression.
Systemic infection occurs when large numbers of pathogenic E coli gain access to the bloodstream from the respiratory tract or intestine.
Bacteremia progresses to septicemia and death.
Colibacillosis in Birds
Signs are non-specific.
Acute septicemia in young birds
Hyperemic and enlarged liver and spleen, fluid in body cavities.
Birds that survive gets fibrinopurulent airsacculitis, pericarditis.
Diagnosis: bacterial culture, PCR
Treatment: ATB not recommended due to resistance, prevention is key.
Colibacillosis in Pigs
Pathogenic E.coli strains are classified into:
Enteropathogenic
Shiga-toxin producing
Enterotoxogenic
Enteroinvasive
Enteroaggregative
Diffusely adherent
Diagnosis: PCR, slide agglutination test

Enterotoxic colibacillosis (post weaning diarrhea)
Most common in young piglets, calves, lambs, and human babies.
It occurs during the first 1-2 weeks after weaning, or after some change in feed or management.
E. coli adhere to mucosa and proliferate in the small intestinal lumen, producing endotoxin (shiga toxin), which can damage the digestive tract.
Clinical signs: include stomach cramps, bloody diarrhea, and maybe fever.
Enterotoxaemia colibacillosis
Caused by toxin- producing strains of E.coli.
This toxin is absorbed into blood and acts in other body parts.
Clinical signs:
Oedema disease in swine: swine may die without preliminary signs, or may show anorexia.
Subcutaneous oedema is common.
Internal signs include hydropericardium, oedema of mesocolonm, gastric tissue and mesenteric lymph nodes.
Septicaemic colibacillosis
Common in calves and other young domestic animals.
It is caused by specific serotypes of E coli that possess virulence factors enabling them to cross mucosal surfaces and produce bacteremia and septicemia.
Invasion occurs primarily through the nasal and oropharyngeal mucosa.
There is a period of subclinical bacteremia that is followed by rapid development of septicemia and death.
In the acute disease, the clinical course is short, and signs are related to development of septic shock.
Clinical signs: Listlessness, depression, poor response to external stimuli, collapse, recumbency, and coma.
The feaces are loose and mucoid, but severe diarrhoea is not seen in uncomplicated cases.
Mortality approaches 100%.
15. Avian mycoplasmosis
Mycoplasmosis is an infectious disease caused by bacteria mycoplasma.
They inhabit moist mucosal surfaces especially of the respiratory tract.
They lack a cell wall, making them naturally resistant to many ATB.
Caused by:
Mycoplasma gallisepticum
Susceptible species:
Chickens, turkeys, and various wild birds
Transmission:
Found in respiratory and ocular secretions, eggs and semen.
Enter the body orally, via respiratory tract or conjuctiva.
Ingestion and inhalation by aerosols.
Clinical signs:
IP: 4-14 days
Some are subclinical, others develop mild to severe respiratory signs
Rales, coughing, sneezing, nasal discharge
Dyspnea, conjunctivitis with frothy ocular exudate
Decreased egg production and some egg abnormalities
Cause rhinitis, trachitis, sinusitis and bronchitis
Diagnosis:
Bacterial isolation, PCR, serology – rapid serum agglutination test
Treatments and preventative:
ATB (b-lactam resistance)
Vaccination, sanitation and disinfection
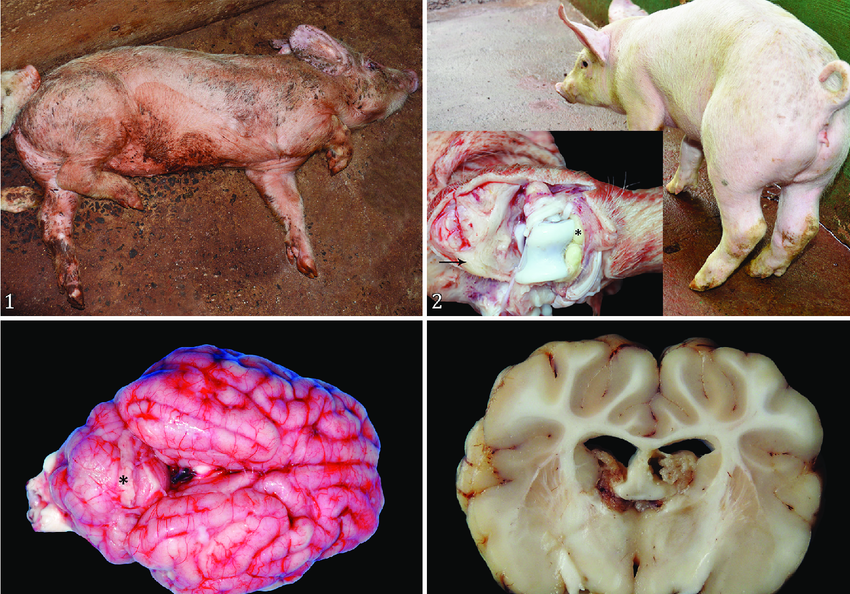
16. Anthrax
Caused by:
Spore-forming bacterium Bacillus anthracis
Susceptible species:
Most common in wild and domestic herbivores
Zoonotic!
Transmission:
Spores can remain infective in soil for many years.
Transmission by inoculation, ingestion, or inhalation.
Grazing animals may become infected when they ingest sufficient quantitates of these spores.
Epizootology:
Epizootics are usually associated with drought, flooding or soil disturbances.
Many years may pass between outbreaks
Spores are relatively resistant to extremes of temperature, chemical disinfection, and dessication.
Clinical signs:
IP: 1-14 days range
Peracute form (most common)
Sudden onset may be only signs.
Rapidly fatal course
Staggering, trembling, dyspnea before collapse, followed by terminal convulsions
Death may occur with only brief evidence of illness
Acute form – ill for a short period, 2 days before they die
Fever
Period of excitement followed by depression, stupor, respiratory or cardiac distress, staggering, convulsions and death
Body temperature may reach 41.5 C
Milk production is materially reduced and pregnant animals may abort
Bloody discharges from the body orifices
Localized, subcutaneous, edematous swelling that can be quite extensive, areas frequent involved are the ventral neck, thorax and shoulders
Horses – acute form
Fever, chills, anorexia, depression, weakness
Severe cholic and bloody diarrhea
Swelling of the neck, sternum, lower abdomen and external genitalia
Death usually occurs within 2-3 days of onset
Pigs:
May develop acute septicemia and sudden death
More usually, a mild chronic form.
Pigs show systemic signs of illness and gradually recover with treatment
Oropharyngeal anthrax is characterized by rapidly progressive swelling of the throat, which may cause death by suffocation
Dogs, cats, and wild carnivores:
Resembles what is seen in pigs.
Wild herbivorous animals:
The expected course of illness and lesions varies by species but resembles, for the most part, anthrax in cattle.
Pathology:
Spores infect macrophages, germinate and proliferate
Lethal toxin and edema toxin are produced
Cause local necrosis and extensive edema, which is a frequent characteristic of the disease
Bacteria multiply in the lymph nodes
Pathological findings:
→ To avoid environmental contamination, post mortem examinations of carcasses of animals suspected to have died of anthrax are discouraged!!
→ Lesions most commonly seen are those of a generalized septicaemia often accompanied by an enlarged spleen having a “blackberry jam” consistency and poorly clotted blood
- Haemorrhage from the nose, mouth, vagina and/or anus at death may be found (bleeding from orifices)
Diagnosis:
Detecting bacteria in blood, take sample from the carcass.
Bacterial culture, PCR, anthrax immunochromatographic test
Treatment and prevention:
Vaccination
quarantine, effective carcass disposal, do not open carcass in the field!!
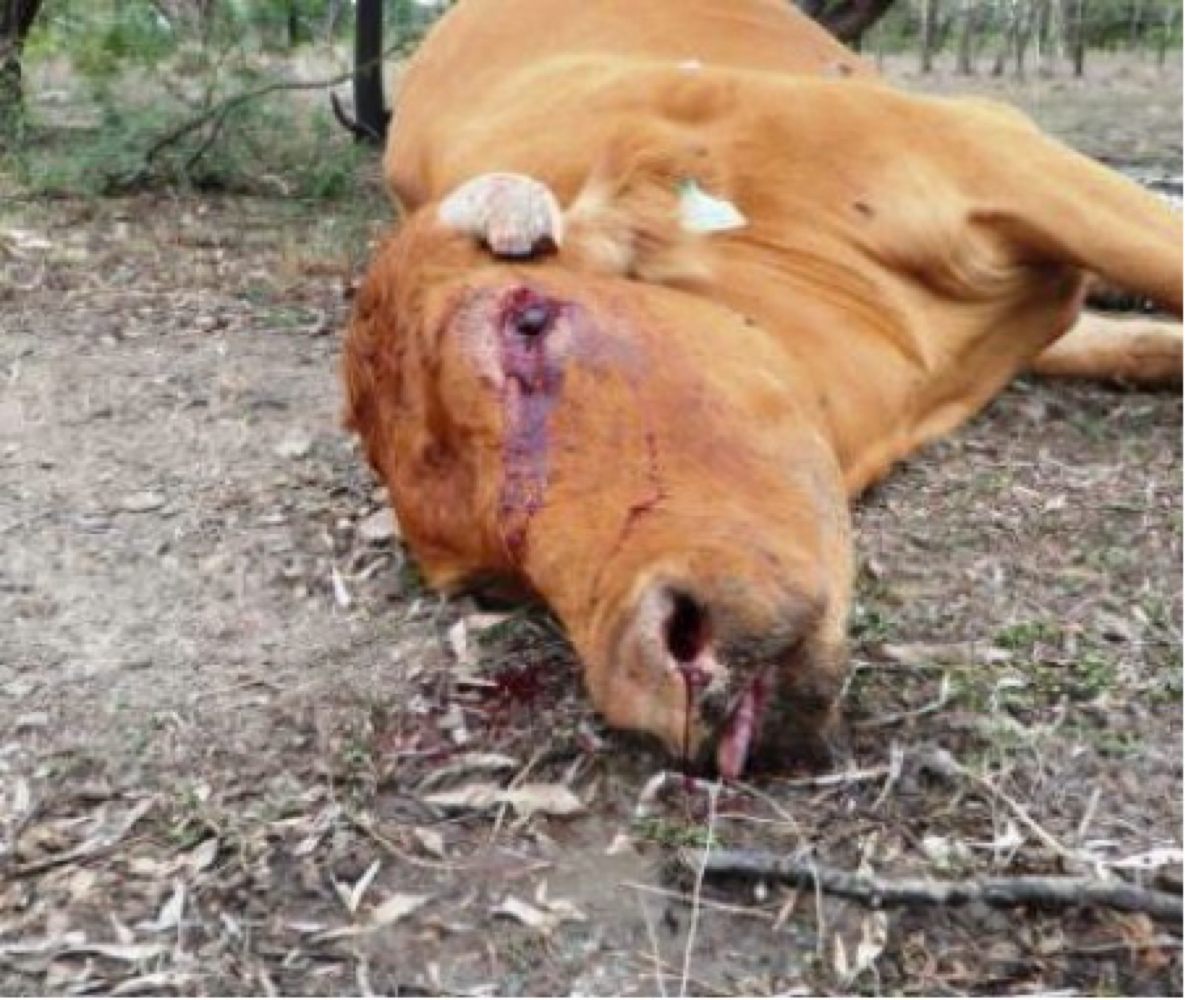
17. Anaerobic infections
Anaerobic infection are caused by bacteria able to cause infections under circumstances with no to little oxygen.
Gram-negative anaerobes and some infections that they cause:
● Bacterioides – neonatal diarrhea, mastitis, abortions in cattle
● Fuscobacterium – stomatitis in swine, necrotic arthritis in ruminants
● Porphyromonas – periodontitis, aspiration pneumonia
● Prevotella – intra-abdominal infection, infection of soft tissues
Gram positive anaerobes and some infections that they cause:
Actinomyces – infectious of heard and neck, abdominal infection, aspiration pneumonia
Clostridium – phlegomonous gastritis, necrotic enteritis, toxicosis
Peptostreptococcus – orqal, respiratory and intra-abdominal infections
Bacillus (facultative anaerobes) – anthrax
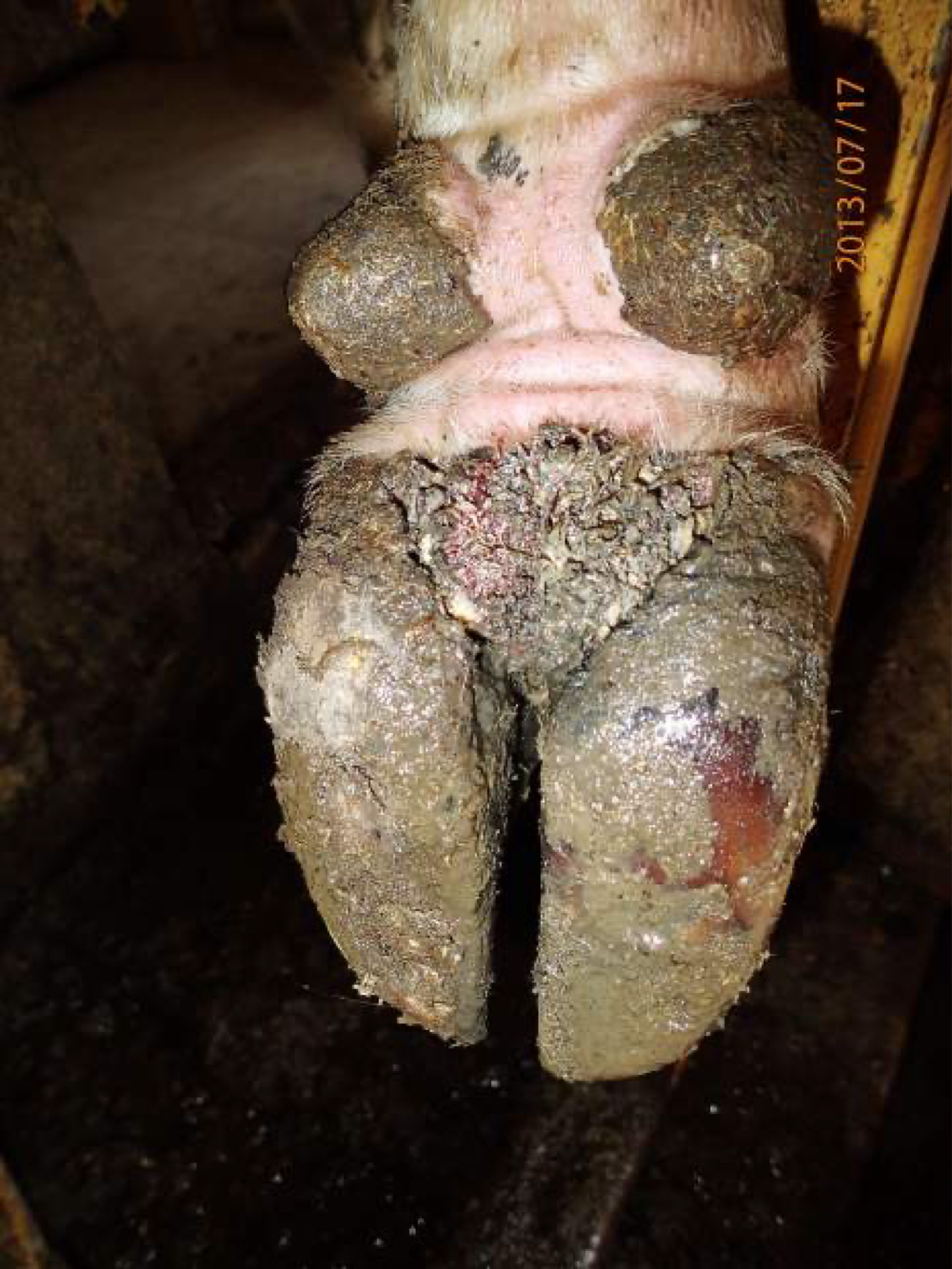
Footrot
Caused by:
Bacteroides nodosus – Sheep and cattle
B. melaninogenicus
Fusobacterium necrophorum – Cattle
Susceptible species:
Sheep and cattle, all ages, worldwide
Transmission:
Introduction from neighboring flocks, direct and indirect transmission.
Reservoirs are subclinical or chronically infected.
Epizootology:
It is a seasonal disease, more common during summer and wet conditions
Clinical signs:
It is an infectious pododermatitis
Lameness
Pain
Rotting odor of the affected parts of the body
Lesions seen in interdigital skin, can spread to the sole
Pathology:
Accompanying pathogens (Fusobacterium) produce leukotoxins that protect Bacterioides from phagocytosis.
Diagnosis:
Clinical examination, lesions and odor.
Based on a scoring system
Treatment
Footbath with 10% zinc sulphate and ATB
Prevention
quarantine of newly introduced animals, clinical examination of new animals, vaccination, suitable zoohygienic conditions, restriction of contact with herds with an unknown health situation.
Necrobacillosis
Caused by:
Fuscobacterium necrophorum → cause mixed infections
Susceptible species:
Member of normal human and animal flora of the mouth, gastrointestinal tract and urogenital tract.
Transmission:
Animal without clinical signs.
Associated with bucket feeding, where buckets are contaminated with faeces.
Bacteria enter through abrasions in the mucosa of the pharynx and larynx
Necrobacillosis in cattle
Calf diptheria
Necrotic laryngitis in cattle.
Bacterial infection of pharynx and larynx
o Oral form:
→ foul smelling ulceration
→ swelling of the cheek and pharyngeal region
→ deep ulcers on the tongue, palate and inside of cheeks
→ high temperature
→ coughing
→ loss of appetite
→ pneumonia
o Laryngeal form:
→ coughing
→ moist and painful
→ high temperature
→ loss of appetite and depression
→ difficult breathing, chewing and swallowing
→ pneumonia.
2. Footrot in cattle (mixed infection with bacteroides nodosus)
3. Necrobacillosis after abortion
4. Necrobaciollsis of umbilical cord
Necrobacillosis in sheep
Footrot in sheep
Lamb diptheria
Omphalophlebitis in lamb
Necrobacillosis in pigs
Piglets dysentery
Footrot in pigs
Necrobacillosis of pig`s snout and head skin
Necronbacillosis of mammary gland in sow
Treatment:
Systemic ATB
18. Clostridial diseases
Characteristics:
Found in the environment as spores
Pathogenic effect is toxins
Diagnostic approach is evidence of infectious agents and toxins in material
Cause alimentary, contagious and traumatic infections
Clostridial diseases are infections caused by the genus Clostridium, known for producing potent toxins that result in severe health issues in various animal species.
Neurotoxic clostridia:
Botulism (Clostridium botulinum)
Tetanus (Clostridium tetani)
Histotoxic clostridia:
C. septicum (Braxy)
C. chauvoei
→ Blackleg
cattle and sheep
edematous and crepitant swellings in the hip, shoulder, chest, back, and neck.
Small swelling, hot, painful → enlarges → cold and insensitive → death
Diagnosis: clinical signs, fluorescent test and PCR
Prevention: vaccination
C. novyi
→ Black disease (infectious necrotic hepatitis)
sheep and cattle
liver flukes
Fecal contamination of pasture in summer
sudden death
grayish-yellow necrotic foci post mortem.
Prevention: active immunization, reduce the occurrence of liver flukes
C. haemolyticum
C. difficile
→ Malignant edema
in all animals, caused by many spp.
Caused by deep wounds
IP is short
soft swellings
dark muscles in affected area
severe toxemia, death
Therapy: surgical treatment of wounds, ATB, hyperimmuneserum
Prevention: vaccines
Enterotoxic clostridia:
Clostridium perfringens
produce entertotoxins
several strains A-E.
→ Enterotoxemia of poultry
Type A and C
chickens, young animals
inflammation and ulceration of jejunum and kidney damage
→ Enteritis in pigs
Type A and C
Affect piglets
Sudden onset of hemorrhagic diarrhea followed by collapse and death – brownish liquid feces.
→ Clostridia-associated enterocolitis in horses
Typically undiagnosed (colitis-X),
non specific clinical signs; diarrhea with or without blood, colic, fever, reduced feed intake, lethargy, high mortality.
Therapy: Metronidazol and supportive care
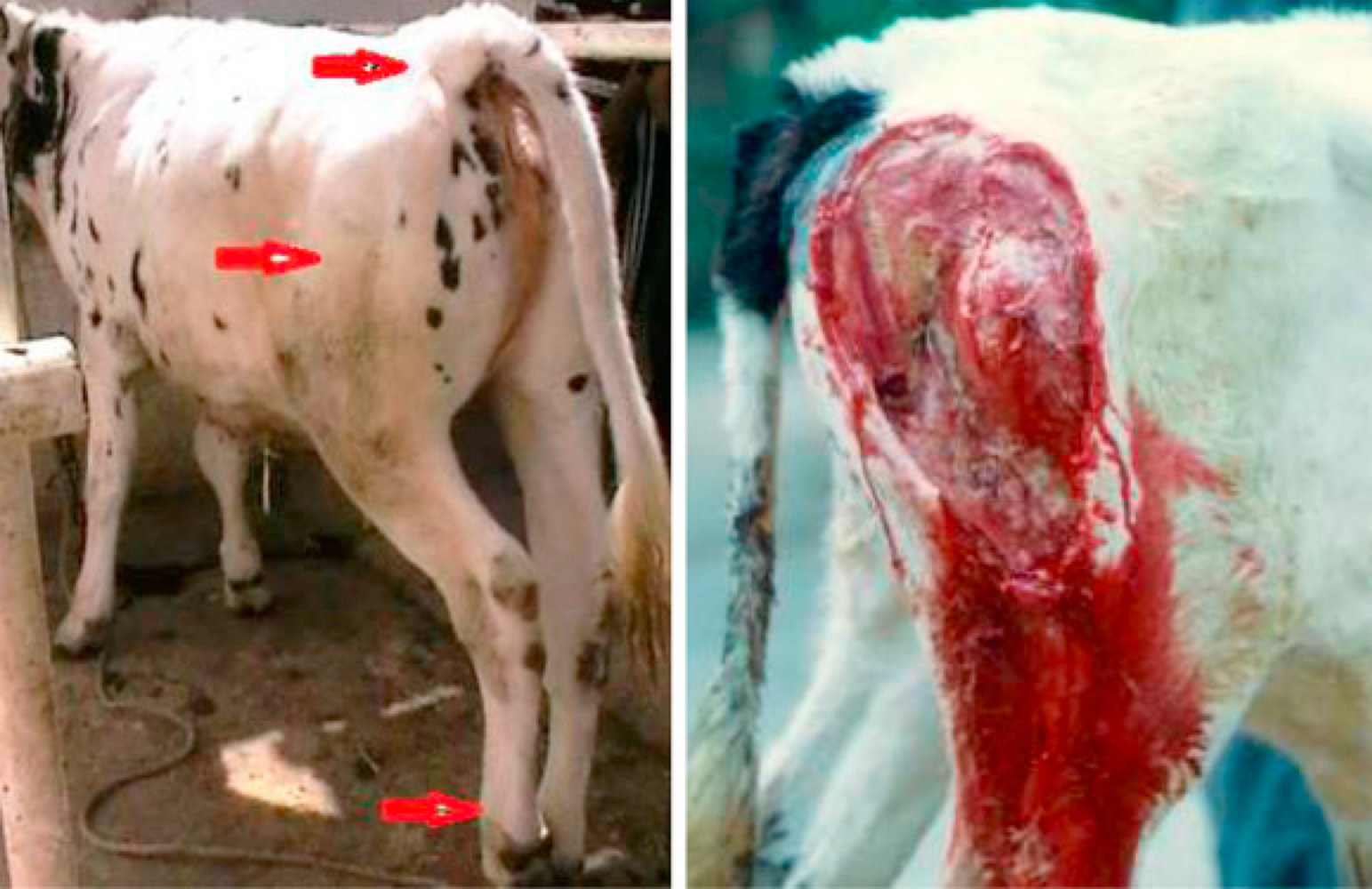
Glanders
Caused by:
Burkholderia mallei
Susceptible species:
Horses, mules and donkeys, and humans. Zoonotic!
Transmission:
Contact with infected horses, most often via respiratory secretions and exudates from skin lesions.
Horses often get infected when ingesting contaminated food or water.
Epizootology:
Eradicated in many countries. Very fatal if untreated!!
Clinical signs:
Can be infected by one or more forms at the same time.
Death occurs rapidly within a few days.
Chronic form can occur in horses.
More severe in donkeys and mules
Nasal form
Deep ulcers and nodules develop inside the nasal passage, cause thick mucopurulent yellowish discharge
Enlarged regional lymph nodes
Can spread to involve lower respiratory tract
Pulmonary form
Most common form
Nodules and abscesses in lungs
Mild to severe respiratory signs; coughing, dyspnea
Fever
Cutaneus form
Nodules on the skin, along the course of lymphatic vessels, typically on legs.
Nodules often rupture and ulcerate, discharging an oily, thick yellow exudate
Ulcers heal slowly
Chronically enlarged regional lymph nodes
Swelling of joints
Humans
Septicemia, pulmonary infection, acute localized infection and chronic disease
Diagnosis:
Bacterial culture
PCR, ELISA
Mallein test (hypersensitivity test)
Treatment and prevention:
ATB
euthanize positive animals.
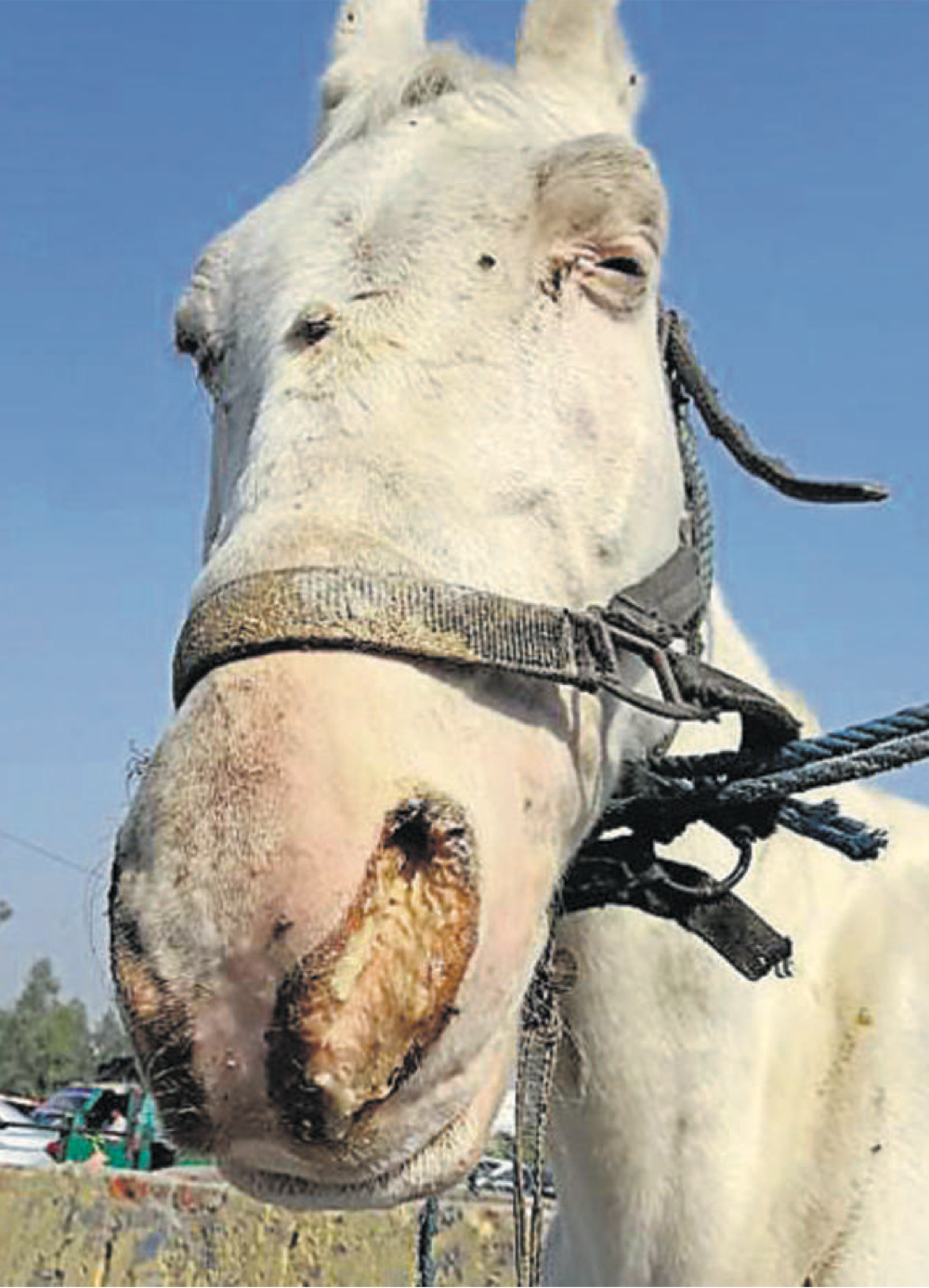
20. Tetanus – Lockjaw
Caused by:
Clostridium tetani
Susceptible species:
In all species
Humans and horses more susceptible
Stivkrampe i menneske!!
Transmission:
Spores of C.tetani are everywhere in the environment, including soil, dust, and manure.
The spores develop into bacteria when they enter the body.
Usually introduced through deep wounds.
Epizootology: Worldwide
Clinical signs:
Serious bacterial disease affecting the nervous system, leading to painful muscle contractions of jaw and neck muscles.
Starts in the jaw and progress to the rest of the body.
Tetanic muscle spasms
Tachycardia and tachypnoea
Fever, sweating, headache, trouble swallowing
Difficult to chew food – lockjaw
Protrusion of third eyelid
80% mortality
Pathology:
Caused by neurotoxins, released when bacteria undergo autolysis.
Toxin binds to acetylcholinesterase, so it is not able to break down acetylcholine → muscular spastic paralysis.
Diagnosis:
Anamnesis and clinical findings.
Tetanus toxin demonstrated in serum
Treatment:
Antitoxin, wound care, penicillin and supportive care.
vaccination

21. Botulism
Caused by:
Clostridium botulinum
Susceptible species:
in all species, more common in horses and ruminants.
Zoonotic!
Transmission:
Ingestion of the organism, neurotoxin or spores, from spoiled stored silage or grain.
Contamination of open wounds with clostridial spores
Inhalation of the neurotoxin is also possible
Epizootology: Worldwide occurrence
Clinical signs:
Characterized by progressive motor paralysis.
In animals it appears as an ascending paralysis that affects the hindlimbs first
difficulties in chewing and swallowing
weakness and incoordination.
Droopy eyelids, dilation of pupils and slow pupillary reflexes.
Death usually results from paralysis of respiratory muscles.
Pathology:
Alimentary infection
bacteria release botulinum neurotoxin → muscle paralysis.
The lethal human dose is 0.0001 mg
Diagnosis:
Anamnesis and clinical signs.
Botulism toxin is identified in environmental samples, serum, GIT content.
ELISA, PCR.
Treatment and prevention:
Antitoxin and supportive care.
Prevention with forage quality and vaccination.

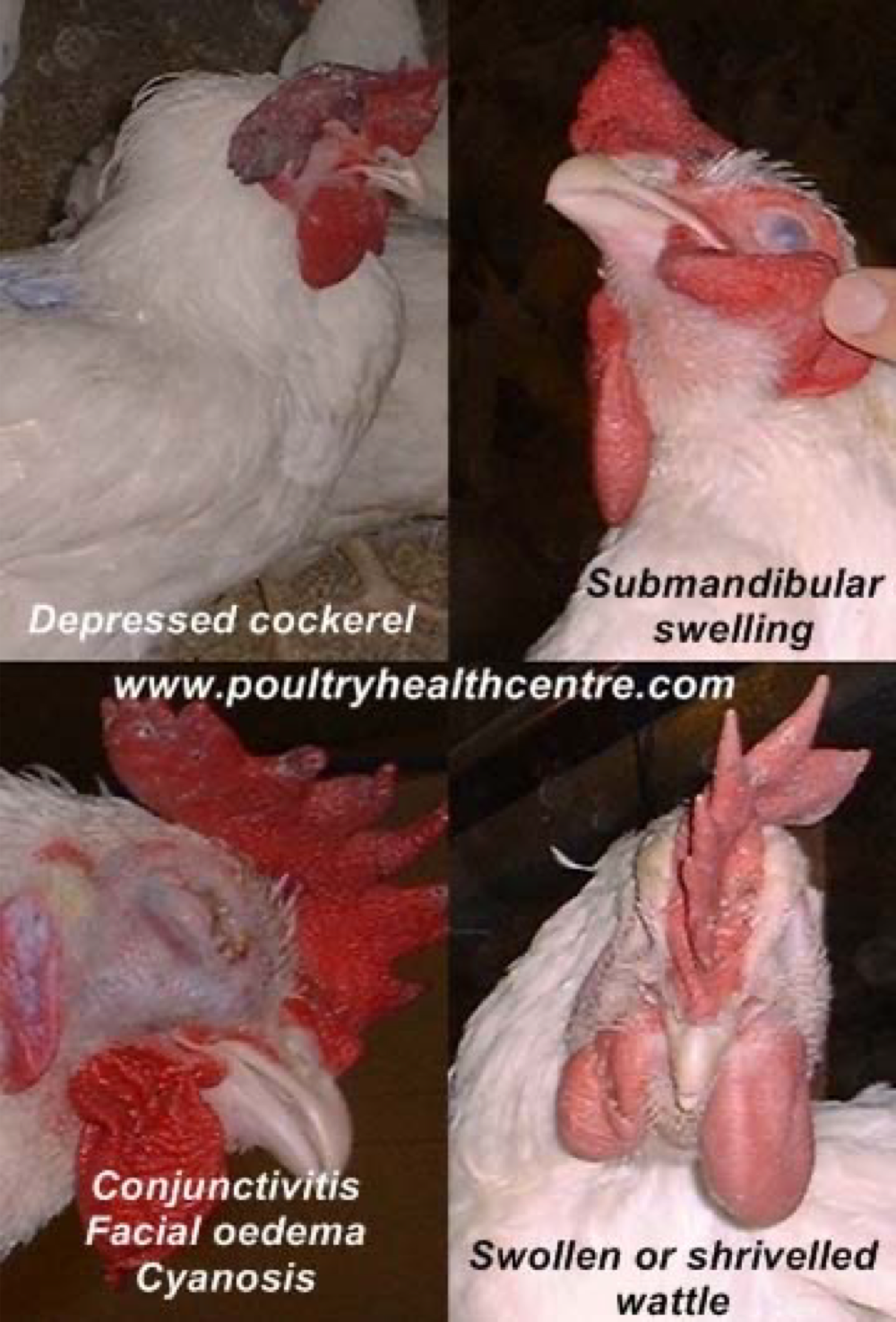
22.Fowl cholera
Caused by:
Pasturella multocida
Susceptible species:
Chickens, turkeys and other birds.
Adult birds and old chickens are more susceptible. Zoonotic!
Transmission:
Oral or nasal with transmission via nasal exudate, feces and contaminated soil, equipment and people.
Epizootology:
Outbreaks occur in cold and wet weather, typically in late summer, fall and winter.
Clinical signs:
2 different forms:
Acute:
dead birds without any clinical signs
fever, depression, anorexia, ruffled feathers, green urates
Mortality increases rapidly.
Chronic:
swollen wattles, joints, tendons and footpads due to accumulated fibrinosuppurative exudate
May be exudative conjunctivitis and pharyngitis
Diagnosis:
Bacterial culture
PCR, ELISA and other serological tests
Treatment and prevention:
ATB may reduce mortality but wont eliminate P. multocida from a flock.
Good biosecurity. Vaccination.