7. Heme Metabolism
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
13 Terms
Porphyrin and Heme Structure
Porphyrins (collection of pyrrole rings) and heme are produced virtually in ____ tissues, but it is more pronounced in the ______ _______ (hemoglobin) and in the _____ (cytochromes)
Attachment of ____ to the _____ of the porphyrin ring gives rise to _____
Heme is largely a _______ structure with the iron slightly _________ ____ of the plane
Heme is a critical component of all _______ chains and is responsible for _________ oxygen
Iron has __ planar bonds coordinated with the __________ of the prophyrin
The 5th bond from the heme Iron is __________ to the plane and binds ____________ _________ of the __________ chain
The 6th bond from the heme iron is ___________ of the plane and is available to bind ___________
Only Iron in the ________ ( ___ ) state will bind oxygen, Iron in the _________ + globin is called ___________
Porphyrin and Heme Structure
Porphyrins (collection of pyrrole rings) and heme are produced virtually in all tissues, but it is more pronounced in the bone marrow (hemoglobin) and in the liver (cytochromes)
Attachment of iron to the center of the porphyrin ring gives rise to heme
Heme is largely a planar structure with the iron slightly sticking out of the plane
Heme is a critical component of all globin chains and is responsible for binding oxygen
Iron has 4 planar bonds coordinated with the nitrogens of the porphyrin
The 5th bond from the heme Iron is perpendicular to the plane and binds histidine imidazole of the globin chain
The 6th bond from the heme iron is adjacent/to the side of the plane and is available to bind oxygen
Only Iron in the ferrous Fe2+ (reduced) state will bind oxygen, Iron in the ferric Fe3+ + globin is called methemoglobin (HbM)
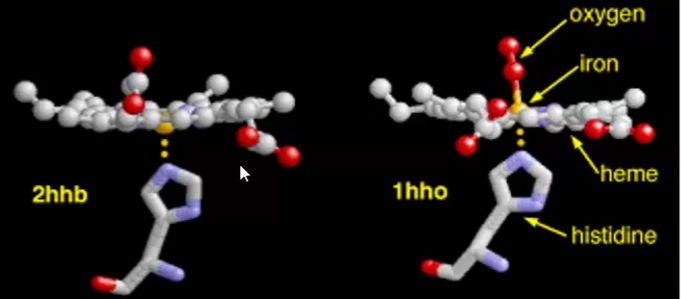
Functions of Heme in:
Hemoglobin
Myoglobin
Cytochrome C
Cytochrome P450
Catalase
tryptophan pyrrolase
Functions of Heme in:
Hemoglobin → oxygen transport in blood
Myoglobin → storage of oxygen in the muscles
Cytochrome C → Involvement in e- transport chain
Cytochrome P450 → Hydroxylation of xenobiotics
Catalase → degradation of hydrogen peroxide
Tryptophan pyrrolase → oxidation of tryptophan
Biosynthesis of heme involves both ______ and _________ reactions and intermediates
Heme biosynthesis occurs in most mammalian cells except __________ _______________ because they lack _____________
Approximately %85 of heme synthesis occurs in ____________ precursors in the _______ __________
Biosynthesis of heme involves both cytosolic and mitochondrial reactions and intermediates
Heme biosynthesis occurs in most mammalian cells except mature erythrocytes because they lack mitochondria
Approximately %85 of heme synthesis occurs in erythroid precursors in the bone marrow
Heme biosynthesis steps
Succinyl CoA + glycine — ALA synthase→ delta-aminolevulinate (ALA)
Rate-limiting step
Pyridoxal phosphate dependent
Occurs in mitochondria and then ALA exits and enters cytosol
two molecules of ALA — ALA dehydratase/porphobilinogen synthase→ Porphobilinogen
4 Porphobilinogen molecules —uroporphyrinogen I synthase → hydroxymethylbilane
Hydroxymethylbilane —uroporphyrinogen III synthase→ Type III Porphyrinogen
OR
Hydroxymethylbilane —spontaneous cyclization → Type I Porphyrinogen (CAUSES PROPHYRIA BC SUBSTITUENTS ARE INVERSE)
TOO MANY STEPS THAT ARE IRRELEVANT IMO, JUST RECOGNIZE THE ORDER:
Coproporphyrinogen II : uroporphyrinogen carboxylase
Protoporphyrinogen III → Enzyme: Coproporphyrinogen oxidase
Protoporphyrin IX → Enzyme: Protoporphyrinogen oxidase
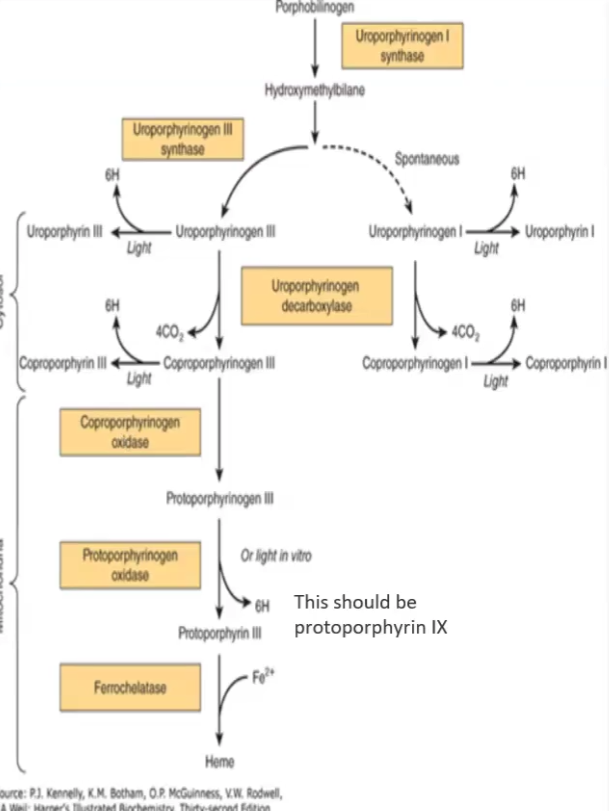
Porphyrinogens are __________ but when they are oxidized they become _________
Porphyrinogens are colorless but when they are oxidized they become colored
ALA synthase 1 (ALA1) vs ALA2 synthase
ALA synthase 1: expressed throughout the body, inhibited by heme
ALA synthase 2: expressed only in erythrocyte precursor cells, inhibited by erythropoetin (produced by kidney)
3 most important Porphyrias
Major symptoms of Porphyrias → “Vampire disease”
Acute Intermittent Porphyria
Congenital Erythropoietic Porphyria
Porphyria cutaneatarda
Mutations in various genes leads to abnormalities in heme synthesis enzymes which leads to
Porphyrinogens → photosensitivity
ALA and PBG accumulation → Neuropsychiatric symptoms and signs
All 3 Porphyrias learned
Defective enzyme
Symptoms
occurrence
Acute Intermittent Porphyria:
Defective Uroporphyrinogen synthase I
Symptoms: Severe abdominal pain, vomiting, confusion, psychosis, seizures
Symptoms caused mostly by ALA and PBG (porphobilinogen) accumulation NOT little heme
Congenital Erythropoietic Porphyria: extremely rare
Defective uroporphynogen synthase III
Dark urine, sunlight sensitivity, erythrodontia/fluorescent teeth, increased hair growth
Porphyria Cutanea Tarda: most common
Defective Uroporphyrinogen decarboxylase
Symptoms: Sensitivity to sunlight and fragility of exposed skin, increased hair growth (especially face)
Plubism
Cause
Symptoms
How is it related to porphyria
Plubism = Lead Poisoning
Strongly inhibits: ALA dehydratase and Ferrochelatase
Mimics Porphyria symptoms: severe abdominal pain, vomiting, fatigue. irritability and developmental delays
Heme Catabolism/Degradation
Where does it take place and by which cells
What components are recycled?
Steps
Takes place in liver, spleen and bone marrow by reticuloendothelial cells
Recycled: iron back to iron poop, globin degraded into constituent AA
Steps:
Heme —heme oxygenase → Biliverdin
Iron and CO2 released
Biliverdin —biliverdin reductase → Bilirubin
NADPH needed
Biliverdin binds to Albumin to travel in the blood and go to the liver
Biliverdin + UDP-gluconic acid - UDP- glucuronosyl transferase→ Bilirubin diglucuronide
Conjugation step
Bilirubin diglucuronide → Bile
When Bilirubin diglucuronide is turned into Bile, the glucuronosyl moiety is removed by intestinal bacterial ______________
Similarly, the fecal flora reacts with ___________ to make them colorless
Most (95%) of the bile is reabsorbed back into the_______ and only a small amount enters _______ and is excreted in the ________ by _________ through the spontaneous oxidization to ________
_________ gives brown appearance to feces
When Bilirubin diglucuronide is turned into Bile, the glucuronosyl moiety is removed by intestinal bacterial beta-glucuronidases
Similarly, the fecal flora reacts with urobilinogens to make them colorless
Most (95%) of the bile is reabsorbed back into the liver and only a small amount enters circulation and is excreted in the urine by kidney through the spontaneous oxidization to urobilin
Stercobilin gives brown appearance to feces
What are the laboratory plasma bile levels for Hyperbilirubinemia and Jaundice/Icterus?
Unconjugated bilirubin vs. Conjugated bilirubin
Hyperbilirubemia >1.0 mg/dL
Jaundice/Icterus > 2.0-2.5 mg/dL
Unconjugated bilirubin (hydrophobic)
Does not appear in urine
Can cross brain-blood barrier and enter CNS, causing Kernicterus (encephalopathy due to hyperbilirubemia)
Moderately unconjugated bilirubin in Pre-hepatic (hemolytic anemias)
Conjugated bilirubin (hydrophilic)
Can appear in urine
Frequent in Hepatic and Post-hepatic (liver diseases and biliary tree obstruction)
Prehepatic, Posthepatic and Hepatic
Pre-hepatic
Caused by acute or chronic hemolytic anemia
Too many hemolyzed RBC’s cannot be turnt into bilirubin fast enough
Unconjugated bilirubin
Increased urobilinogen and stercobilinogen
Hepatic (Liver Disease)
Caused by Hepatocellular disturbances (cirrhosis and hepatitis), Neonatal/physiologic jaundice, Gilbert syndrome (benign), Crigler-Najjart, Bilirubin transport disturbance (Dubin-Johnson Syndrome)
Post-hepatic obstruction:
Caused by diseases that obstruct the bile duct
Conjugated bilirubin
Decreased urobilinogen, increased bilirubin diglucuronide
White feces because no stercobilin
Bile Duct Obstruction