Lesson 5.2 Acid–base titrations
0.0(0)
Card Sorting
1/9
There's no tags or description
Looks like no tags are added yet.
Last updated 11:47 PM on 1/24/26
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
10 Terms
1
New cards
What is a titration curve and how is it set up?
A graph of pH (y-axis) vs volume of titrant added (x-axis). The equivalence point is at the MIDDLE of the steepest section of the curve
2
New cards
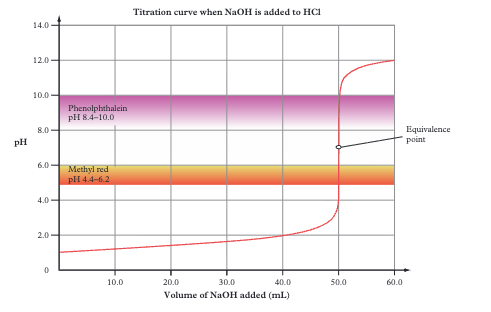
Strong acid + strong base titration curve features
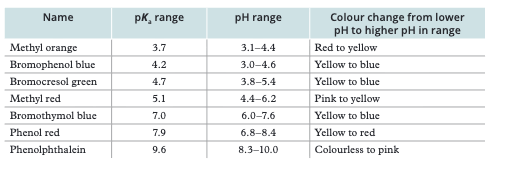
Starts at low pH, ends at high pH. Equivalence point at pH 7. Very steep section (pH 3–11). Almost any indicator works (methyl red or phenolphthalein)
3
New cards
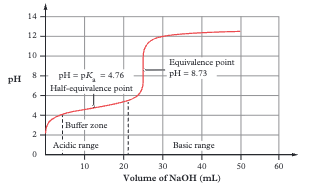
Weak acid + strong base titration curve features
Starts at low pH (but higher than strong acid). Has BUFFER REGION (gradual pH change). Equivalence point at pH > 7. Use phenolphthalein (pH 8–10)
4
New cards
Why is the equivalence point above pH 7 for weak acid + strong base?
At equivalence, all acid has reacted leaving only the CONJUGATE BASE (e.g., CH₃COO⁻), which is itself a weak base and makes the solution basic
5
New cards
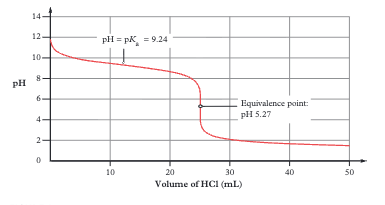
Strong acid + weak base titration curve features
Starts at high pH (~12), decreases. Has buffer region. Equivalence point at pH < 7. Use methyl orange or methyl red (pH 3–7)
6
New cards
Why is the equivalence point below pH 7 for strong acid + weak base?
At equivalence, all base has reacted leaving only the CONJUGATE ACID (e.g., NH₄⁺), which is itself a weak acid and makes the solution acidic
7
New cards
What is the buffer region on a titration curve?
The relatively FLAT section where pH changes very gradually. Occurs when both weak acid AND its conjugate base are present in significant amounts — resists pH change
8
New cards
What is the half-equivalence point and what is its significance?
The point where HALF the analyte has reacted (volume = half of equivalence volume). At this point: pH = pKa (for weak acid) or pOH = pKb (for weak base)
9
New cards
How do you determine pKa from a weak acid titration curve?
Find the half-equivalence point (halfway to equivalence volume). Read the pH at this point — this equals pKa. Then Ka = 10⁻ᵖᴷᵃ
10
New cards
How do you determine pKb from a weak base titration curve?
At half-equivalence point, pOH = pKb. Find pH at half-equivalence, then pKb = 14 − pH. Then Kb = 10⁻ᵖᴷ